|
Coupling Single Platinum Atoms to Form Clusters Improves Catalytic Activity
March 2020
Environmental and sustainability issues demand that new motor vehicles produce lower emissions while increasing fuel economy. These goals 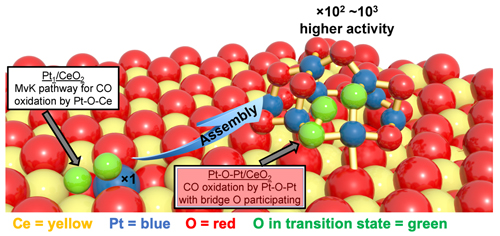 require improvements to many vehicle systems, including the catalytic converter. Catalytic converters reduce noxious emissions, chiefly by converting carbon monoxide (CO), hydrocarbons, and nitrous oxides (NOx) into carbon dioxide (CO2), nitrogen, and water. In many converters, this task is achieved by mating platinum-group metals with cerium oxide (CeO2)-based materials. An exciting development in recent years has been the appearance of single-atom catalysis, which greatly reduces the amount of expensive platinum (or similar metal) that is needed. In the single-atom approach, individual platinum atoms are dispersed throughout a matrix of CeO2. Now, a multi-institution collaboration of researchers has demonstrated that enhancing the single-atom approach can lead to vastly greater catalytic activity. The researchers formed small clusters of platinum (Pt) and oxygen atoms by coupling the 'seeds' of isolated platinum atoms. Under low-temperature conditions (<150° C) the platinum atoms in these tiny clusters exhibited up to 1000 times the catalytic activity of individual platinum atoms... require improvements to many vehicle systems, including the catalytic converter. Catalytic converters reduce noxious emissions, chiefly by converting carbon monoxide (CO), hydrocarbons, and nitrous oxides (NOx) into carbon dioxide (CO2), nitrogen, and water. In many converters, this task is achieved by mating platinum-group metals with cerium oxide (CeO2)-based materials. An exciting development in recent years has been the appearance of single-atom catalysis, which greatly reduces the amount of expensive platinum (or similar metal) that is needed. In the single-atom approach, individual platinum atoms are dispersed throughout a matrix of CeO2. Now, a multi-institution collaboration of researchers has demonstrated that enhancing the single-atom approach can lead to vastly greater catalytic activity. The researchers formed small clusters of platinum (Pt) and oxygen atoms by coupling the 'seeds' of isolated platinum atoms. Under low-temperature conditions (<150° C) the platinum atoms in these tiny clusters exhibited up to 1000 times the catalytic activity of individual platinum atoms...
|
|
Why Z Phase Matters
December 2019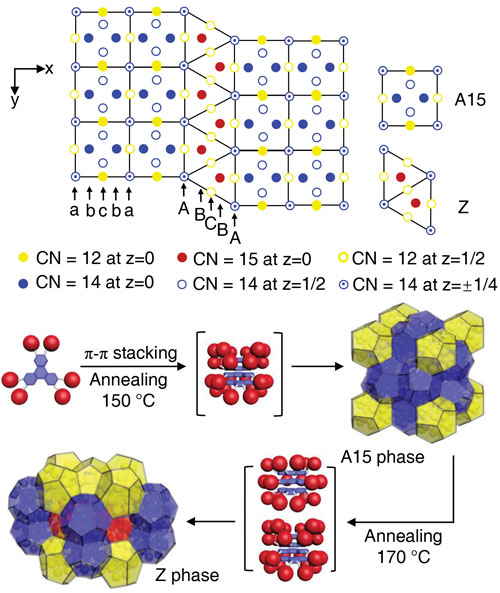
Deep down on the inside, at the level of atoms, most solid pieces of matter are crystalline. Pure metals like iron often have simple crystal structures, while others can be more complex. Some are even quasicrystals, which have their atoms in complicated, crystal-like patterns that never quite repeat. Studies of the structure of crystalline materials show that many factors influence that atomic geometry, but the size and shape of an atom or molecule is the most important. And that can help solve real-world problems. Famously, British engineer Lord Kelvin in 1887 asked how space could be most efficiently partitioned into cells of equal volume with the least area of surface between them. Kelvin’s problem has never been definitively solved, but in 1994 mathematicians demonstrated that a crystal structure, the Frank Kasper A15 phase, was more efficient than any previous solution. Frank Kasper structures are ordered approximations of quasicrystals. The A15 phase was the inspiration for the Beijing National Aquatics Center water cube for the 2008 Olympics because it allowed for the construction of a space with cells of equal volume using the least amount of material. Another Frank Kasper structure, the Z phase, could theoretically construct the same space with even less material—if the cells are allowed to be unequal. Now, a team of scientists has used the U.S. Department of Energy’s Advanced Photon Source (APS) to show that the Z phase can exist in a soft, self-assembling material. Their work provides insight into potential special properties of Z phase matter, and shows how other, more complex Frank Kasper phases could be constructed.
In order to build a material with a Frank Kasper Z phase structure, researchers from the University of Akron in Ohio, the South China Institute of Technology, Northern Illinois University, The University of Tokyo (Japan), and the Riken Center for Emergent Matter Science (Japan) had to build a nano-sized, multipart molecule. They attached six identical polyhedral silesquioxane cages (six-sided polygons made of silicon and oxygen) to a central triphenylene core using covalent links with adjustable lengths. By tuning the lengths of the links just right, the researchers could persuade the complex molecules to self-assemble into spherical motifs, which then squish together to form polyhedra in Frank Kasper patterns (Fig. 1).
|
|
Ultraviolet Light Makes a Polymer Run Hot or Cold
October 2019
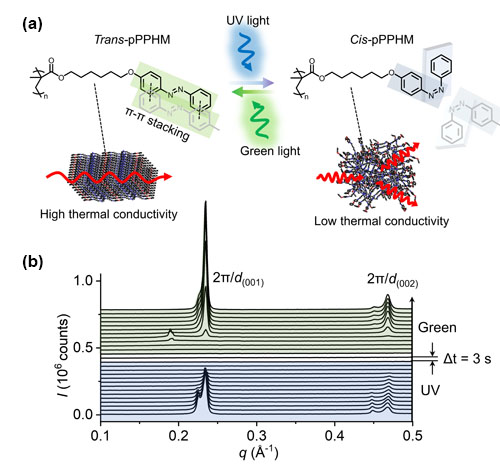
Plastic materials that can be switched from their normal character as heat insulating materials to ones that can conduct heat well by simply turning the lights on could have a wide range of applications in engineering. Applications might be found in carrying and releasing drug molecules to disease sites in the body, or controlling the way in which components in a device stick together. Or perhaps more obviously, in allowing heat to be channeled through a building or vehicle in a controlled way without the need for louvers and fans. Toward that end, researchers from the University of Illinois at Urbana–Champaign, Argonne National Laboratory, and the Air Force Research Laboratory used the U.S. Department of Energy’s Advanced Photon Source (APS) to investigate a plastic, polymeric material with a view to modifying it to change properties reversibly when light shines on it.
The properties of countless materials can be changed by heating them, adding acid or alkali, or in the case of so-called “photoresponsive materials,” by bathing them in light. Such materials have found utility in diverse areas of modern technology for controlled drug delivery, in diagnostic and environmental sensors, in self-healing devices, and in the development of artificial muscles for robotics and other types of actuators. One aspect of such control that has been a prime target for research is the development of materials that can be switched from heat insulators to conductors and back again with a simple external stimulus.
|
|
Controlling Thermal Conductivity of Polymers with Light
March 2019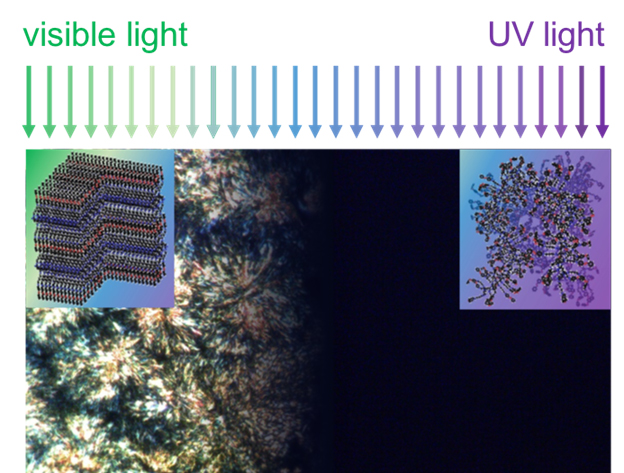
Polymers are regularly used as thermal insulators for everything from keeping beverages hot to keeping sensitive electronics cool. In some cases, polymers can even be used as thermal conductors to enable efficient heating or cooling. In a new study supported by synchrotron x-ray studies at the U.S. Department of Energy’s Advanced Photon Source (APS), researchers from the University of Illinois at Urbana-Champaign, with colleagues from the Air Force Research Laboratory and Argonne National Laboratory, have designed and demonstrated a novel type of polymer demonstrating a switchable thermal conductivity controlled by light. The material has the potential to route the conduction of heat on-demand and enable new, smarter, ways to manage heat.
"Polymers are used extensively in engineered systems, but these materials have almost always been considered thermally static. Discovery of polymers that can be optically triggered to quickly switch between thermally conducting and insulating states will open up entirely new opportunities in thermal engineering,” said Paul Braun, a materials science and engineering (MatSE) professor and director of the University of Illinois Materials Research Laboratory and co-author of the journal article.
|
|
Unexpected Nanoclusters in Lithium-Ion Battery Electrolytes
March 2019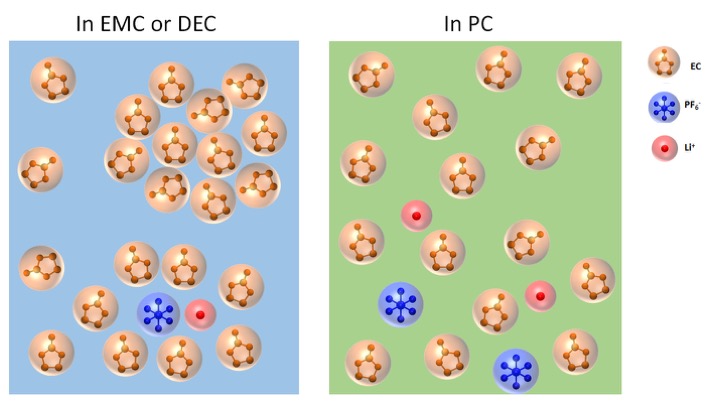
Commercially available since the 1970s, the lithium-ion (Li-ion) battery is the workhorse power source for a variety of applications, including cell phones, laptops, and electric vehicles. However, the basic science underlying how these batteries work at the atomic and molecular levels remains incompletely understood, preventing the technology from reaching its full potential. Lithium-ion batteries are comprised of positive electrodes (anodes), negative electrodes (cathodes), and an electrolyte. The electrolyte’s main role is to act as a conductive pathway for the movement of electricity (in the form of lithium ions, in this case) passing from the anode to the cathode during discharge, allowing the battery to operate. Researchers using the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) at Argonne National Laboratory made a surprising discovery about the complexity of the interactions between different components of the electrolyte, one of the challenges that is set out to be studied in the DOE-funded Argonne-led Joint Center for Energy Storage Research program over the next five years. The fundamental understanding gained from such a study may one day help enhance battery performance.
|
|
Giant Molecules as a Unique Platform for Unconventional Nanostructures
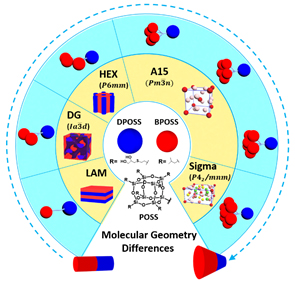 June 2018 June 2018
The engineering of materials into ordered structures is always a critical step to transfer and amplify microscopic molecular functionalities to macroscopic material properties. Tremendous efforts have been made to study the relationship between molecules and the corresponding supramolecular structures as the base for further study of structure-property relationship. Giant molecules are a family of macromolecules with well-defined three-dimension structures built up from molecular nanoparticles with precise chemical structures. They typically include folded globular proteins, polyhedral oligomeric silsesquioxane (POSS), fullerene (C60), and polyoxometalates (POMs). A multitude of studies have demonstrated that they are a unique and efficient platform upon which to construct various ordered patterns in a desired feature size. Recently, researchers using the U.S. Department of Energy’s Advanced Photon Source (APS) have shown that altering the composition and sequence of giant molecules answers some fundamental questions about how giant molecules can be used for the assembly of controllable, well-defined, self-assembled nanostructures.
As shown in Fig. 1, a set of giant molecules were constructed by linking one hydrophilic polyhedral oligomeric silsesquioxane nanoparticle (DPOSS, 14 hydroxyl groups functionalized POSS) with different numbers (n) of hydrophobic POSS(s) (BPOSS, 7 isobutyl groups functionalized POSS). The self-assembly study shows that a supramolecular structure formation sequence ranging from lamella (LAM), double gyroid (DG), hexagonal closed packed cylinders (HEX), to Frank-Kasper (F-K) A15, and further to F-K Sigma phase could be observed by turning the number of hydrophobic BPOSS (see the structure cartoons in Fig. 1).
|
Determining the Absorption Bandgap Inhomogeneity of PbSe Quantum Dots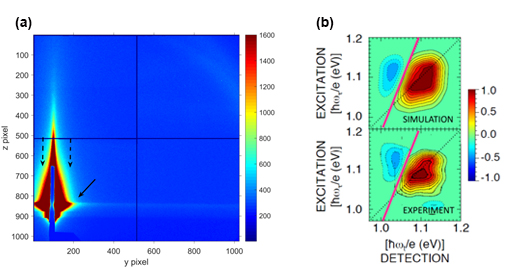
March 2018
Scientists using the U.S. Department of Energy’s Advanced Photon Source (APS) have developed a novel means of determining the absorption bandgap inhomogeneity of colloidal lead selenide (PbSe) quantum dots (QDs) using femtosecond two-dimensional (2-D) Fourier transform spectroscopy. The simple analysis promises to be applicable to solutions and arrays of many other quantum-confined materials as well.
For instance, due to their size-tunable optical and electronic properties, quantum-confined semiconductor nanocrystals have shown great potential in many emerging optoelectronic applications including light-emitting diodes, lasers, solar cells, and photodetectors. However, these and other applications require very stringent control over the line width of the first exciton (electron-hole) transition of the QDs.
|
|
Probing He bubbles in naturally aged and annealed δ-Pu alloys using ultra-small-angle x-ray scattering
January 2018
The self-irradiation of Pu alloys generates He that is trapped within the metal matrix in the form of He bubbles. The distribution of these He bubbles in δ-phase Pu-Ga alloys exhibits a peak near a radius of 0.7 nm, and this size is remarkably stable as function of time. When annealed, the He bubbles in δ-Pu alloys grow, coarsening the distribution. However, the magnitude of this coarsening is uncertain, as different experimental methods reveal bubbles that differ by at least one order of magnitude. Small-angle x-ray scattering results, which can probe a wide range of bubble sizes, imply only a mild coarsening of the He bubble distribution for an annealing treatment of 425 ∘C for 24 h, and analysis of the He bubble content suggests that He is actually lost from the bubbles with annealing.
|
|
A New Catalyst for Making Fuels from Shale Gas
January 23, 2018
In a new study, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory, Tufts University and Oak Ridge National Laboratory teamed up to explore the potential of rhodium-based catalysts for this conversion under milder conditions. “Our work shows the potential of rhodium to enable this conversion under ‘mild conditions’ such as lower temperatures,” said Argonne X-ray scientist Sungsik Lee. “Converting methane to methanol under mild conditions could have significant applications and present a breakthrough in catalysis.”
|
|
Making fuel out of thick air
December 7, 2017
In a new study, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory, Tufts University and Oak Ridge National Laboratory teamed up to explore the potential of rhodium-based catalysts for this conversion under milder conditions. “Our work shows the potential of rhodium to enable this conversion under ‘mild conditions’ such as lower temperatures,” said Argonne X-ray scientist Sungsik Lee. “Converting methane to methanol under mild conditions could have significant applications and present a breakthrough in catalysis.”
|
|
Chemical “dance” of cobalt catalysis could pave way to solar fuels
June 2, 2017
By splitting a water molecule into two atoms of hydrogen and one of oxygen, scientists can use the boundless energy of the sun to make a clean fuel. In a new study from the U.S. Department of Energy’s (DOE) Argonne National Laboratory and Harvard University, scientists have for the first time been able to see an especially important step in the water-splitting process, which may bring us closer to abundant solar energy for all.
|
|
Tunable Amorphous Photonic Materials with Pigmentary Colloidal Nanostructures
January 31, 2017
Amorphous photonic structures using pigmentary α-Fe2O3/SiO2 core–shell nanoparticles are succesfully fabricated. The resulting non-iridicent brilliant colors can be manipulated by shell thickness, particle concentration, and external electrical stimuli using electrophoretic deposition process. Fully reversible and instantaneous color changes as well as noticeable difference between transmitted and reflected colors is...
|
|
Technique improves the efficacy of fuel cells
May 16, 2016
Solid oxide fuel cells, which rely on low-cost ceramic materials, are among the most efficient and promising type of fuel cell. Now, researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), Rensselaer Polytechnic Institute, SciEnergy Systems, Purdue University and the U.S. Department of Energy’s Argonne National Laboratory have found a way to harness the quantum behavior of these fuel cells to make them even more efficient and robust. In doing so, they’ve observed a new type of phase transition in an oxide material.
|
|
Researchers create first entropy-stabilized complex oxide alloys
September 29, 2015
Researchers from North Carolina State University and Duke University have created the first entropy-stabilized alloy that incorporates oxides – and demonstrated conclusively that the crystalline structure of the material can be determined by disorder at the atomic scale rather than chemical bonding.
The researchers then used the Advanced Photon Source at Argonne National Laboratory and X-ray fluorescence spectroscopy to determine that the constituent atoms in the entropy-stabilized oxide were evenly distributed and that their placement in the crystalline lattice structure was random.
|
|
Control of Light with DNA-Nanoparticle Crystals
April 13, 2015
DNA enables one to precisely place nanoparticles into periodic structures (called “superlattices”) in two or three dimensions as either large films or near-perfect single crystals. By constructing the superlattices from gold nanoparticles, a Northwestern University research group, carrying out studies at the U.S. Department of Energy’s Advanced Photon Source (APS), reported in Nature Nanotechnology that they can precisely control how light flows though and interacts with these materials.
|
|
Layered Nanostructure Held Together by DNA
March 18, 2014
Dreaming up nanostructures that have desirable optical, electronic, or magnetic properties is one thing. Figuring out how to make them is another. A new strategy uses the binding properties of complementary strands of DNA to attach nanoparticles to each other and builds up a layered thin-film nanostructure through a series of controlled steps. Investigation at the U.S. Department of Energy Office of Science's Advanced Photon Source has revealed the precise form that the structures adopted, and points to ways of exercising still greater control over the final arrangement.
|
|
Water-Like Properties of Soft Nanoparticle Suspensions
November 25, 2013
This discovery, which is the first instance of experimental observation of such behavior in a colloidal suspension, allows for an extension of the toolbox of the experimental physicist interested in employing suspensions to mimic molecular liquids, with the added advantage of readily accessible length and time scales.
|
|
A Novel Nanobio Catalyst for Biofuels
August 27, 2012
Researchers working at U.S. Department of Energy facilities at Argonne National Laboratory including the Advanced Photon Source, have synthesized and characterized monodisperse gold-core silver-shell nanoparticles using a bio-template that has potential as a water soluble catalyst for creating fuel from biomass such as dead trees, branches and tree stumps, yard clippings, wood chips, and even municipal solid waste
|
|
Velcro for Nanoparticles
November 17, 2010
DNA can do more than direct how bodies are made. It can also direct the composition of many kinds of materials, according to a new study from the U.S. Department of Energy’s Advanced Photon Source at Argonne.
|
|