 Environmental and sustainability issues demand that new motor vehicles produce lower emissions while increasing fuel economy. These goals require improvements to many vehicle systems, including the catalytic converter. Catalytic converters reduce noxious emissions, chiefly by converting carbon monoxide (CO), hydrocarbons, and nitrous oxides into carbon dioxide (CO2), nitrogen, and water. In many converters, this task is achieved by mating platinum-group metals with cerium oxide (CeO2)-based materials. An exciting development in recent years has been the appearance of single-atom catalysis, which greatly reduces the amount of expensive platinum (or similar metal) that is needed. In the single-atom approach, individual platinum atoms are dispersed throughout a matrix of CeO2. Now, a multi-institution collaboration of researchers has demonstrated that enhancing the single-atom approach can lead to vastly greater catalytic activity. The researchers formed small clusters of platinum (Pt) and oxygen atoms by coupling the 'seeds' of isolated platinum atoms. Under low-temperature conditions (<150° C) the platinum atoms in these tiny clusters exhibited up to 1000 times the catalytic activity of individual platinum atoms. The clusters were characterized using various techniques, including x-ray absorption spectroscopy (XAS) performed at the Department of Energy’s (DOE’s) Advanced Photon Source (APS). The innovative approach demonstrated in this study could lead to improved catalytic converters for the next generation of ultra-low-emission vehicles.
Environmental and sustainability issues demand that new motor vehicles produce lower emissions while increasing fuel economy. These goals require improvements to many vehicle systems, including the catalytic converter. Catalytic converters reduce noxious emissions, chiefly by converting carbon monoxide (CO), hydrocarbons, and nitrous oxides into carbon dioxide (CO2), nitrogen, and water. In many converters, this task is achieved by mating platinum-group metals with cerium oxide (CeO2)-based materials. An exciting development in recent years has been the appearance of single-atom catalysis, which greatly reduces the amount of expensive platinum (or similar metal) that is needed. In the single-atom approach, individual platinum atoms are dispersed throughout a matrix of CeO2. Now, a multi-institution collaboration of researchers has demonstrated that enhancing the single-atom approach can lead to vastly greater catalytic activity. The researchers formed small clusters of platinum (Pt) and oxygen atoms by coupling the 'seeds' of isolated platinum atoms. Under low-temperature conditions (<150° C) the platinum atoms in these tiny clusters exhibited up to 1000 times the catalytic activity of individual platinum atoms. The clusters were characterized using various techniques, including x-ray absorption spectroscopy (XAS) performed at the Department of Energy’s (DOE’s) Advanced Photon Source (APS). The innovative approach demonstrated in this study could lead to improved catalytic converters for the next generation of ultra-low-emission vehicles.
The first catalytic converters first appeared in cars in the mid-1970s. While many different materials have since been used, Pt and related metals, such as palladium and rhodium, remain among the most popular catalytic elements. One drawback of these metals is their limited supply and high cost. Traditionally, the platinum-group metals in catalytic converters exist in the form of nanoparticles. This inevitably causes a significant waste because most of the metal atoms are buried inside the nanoparticles and cannot participate in catalysis.
In the early 2000s, scientists serendipitously discovered how to disperse single platinum atoms throughout cerium oxide (commonly called ceria) and other materials. The resulting single-atom catalyst allows all the platinum present to participate, so much less of the precious metal is required. Even though the single-atom catalyst utilizes all the available platinum, the researchers in this study considered whether coupling the isolated and dispersed platinum atoms to form clusters would substantially multiply their overall effectiveness. This hypothesis was tested by preparing and testing both single-atom and clustered platinum systems.
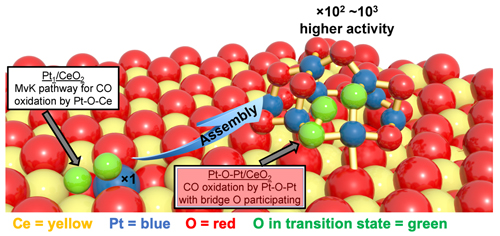
A single-atom catalytic system was produced by dispersing platinum atoms throughout a ceria matrix. The resulting platinum single-atom system is denoted Pt/CeO2. To produce the clustered system, single platinum atoms were used as seeds to generate a much more active Pt cluster through a facile activation treatment, wherein a mild hydrogen gas (H2) reduction was followed by CO-plus-O2 treatment. In the resulting Pt clusters (~1 nanometer in diameter without stacked layers), an oxygen atom is juxtaposed between a pair of platinum atoms (Pt-O-Pt). The platinum/oxygen/ceria unit is denoted Pt-O-Pt/CeO2. The two types of catalytic units and their relative positions to the cerium oxide layer are illustrated in Fig. 1.
The number of platinum and oxygen atoms in a typical cluster was determined using sophisticated computer modeling and various imaging methods. One such method consisted of the XAS measurements performed at X-ray Science Division x-ray beamline 12-BM of the APS, an Office of Science user facility at Argonne National Laboratory. The XAS measurements helped determine that the chemical formula Pt8O14 best reflected the relative numbers of platinum and oxygen atoms in the clusters. In addition, aberration-corrected high-angle annular dark-field scanning transmission electron microscopy images of Pt species were obtained at the DOE’s Advanced Microscopy Laboratory at Oak Ridge National Laboratory (ORNL).
In testing the relative effectiveness of the two systems, the researchers focused on CO oxidation, i.e., the conversion of carbon monoxide to carbon dioxide. For temperatures between 80° C and 150° C, the Pt-O-Pt/CeO2 clusters exhibited up to 1000 times greater catalytic activity in oxidizing CO. Analysis further indicated that the oxygen participating in the CO-to-CO2 conversion came from the Pt-O-Pt layer and not the underlying ceria. This is in stark contrast to conventional reaction pathways, where oxygen is contributed by CeO2.
The vastly improved oxidation of CO at lower temperatures achieved by the platinum clusters has important ramifications for the low-temperature period following cold-engine start, during which current catalytic converters are not being adequately heated to purify the noxious exhaust emissions. Furthermore, the methods used in this research can readily be applied to other catalytic systems; for instance, the platinum clusters can be dispersed on substrates other than ceria. Also, the size of the clusters can be tuned to adapt their catalytic activity and selectivity to better fit different emission environments. ― Philip Koth
See: Hui Wang1, Jin-Xun Liu2, Lawrence F. Allard3, Sungsik Lee4, Jilei Liu5, Hang Li1, Jianqiang Wang1, Jun Wang1, SeH. Oh6, Wei Li6, Maria Flytzani-Stephanopoulos5, Meiqing Shen1, Bryan R. Goldsmith2, and Ming Yang6, “Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms,” Nat. Commun. 10, 3808 (2019). DOI: 038/s41467-019-11856-9
Author affiliations: 1Tianjin University, 2University of Michigan, 3Oak Ridge National Laboratory, 4Argonne National Laboratory, 5Tufts University, 6General Motors Global Research and Development
Correspondence: *[email protected], **[email protected], ***[email protected]
The work from Tianjin University was supported by the National Key R&D Program (2017YFC0211303), the Natural Science Foundation of China (Grant No. 21576207), and the academic collaboration with GM Global R&D. The computational work was supported by start-up funds provided by the University of Michigan. Microscopy work at ORNL was supported by a Strategic Partnership Project funded by GM Global R&D, and, in part, by the U.S. DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office, Propulsion Materials Program. This research used resources of the APS, a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357.
The APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
