The ultimate power source that powers the stars themselves, nuclear fusion is capable of producing clean, essentially unlimited energy and solving humankind's needs into the far distant future if the technological challenges to its realization can be met. Along with achieving the necessary extremely high temperatures and containing the hydrogen plasma fuel, there's the lesser-known problem of devising reactor structural materials that can withstand such heat and radiation conditions. This includes the ability to tolerate the vast amounts of helium produced in the transmutation process, which accumulates inside the polycrystalline reactor materials. Over time, this causes them to become brittle, possibly resulting in breakdown and catastrophic structural failure.
To address this issue, a group of researchers from MIT, Clemson University, the University of Houston and institutions in Turkey and South Korea performed a series of experiments demonstrating that some nano-heterophases of certain materials can store helium securely within their bulk lattices, effectively mitigating its accumulation at grain boundaries and preventing it from causing premature failure. Their work was published in Acta Materialia.
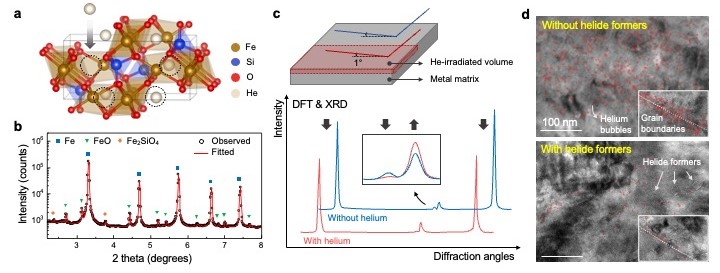
Materials chosen for fusion reactors need to be able to withstand up to a few hundred displacements per atom (dpa) of radiation damage, as well as a few thousand atomic parts per million (appm) of transmutation atoms such as helium. However, the extremely high temperatures to which they are subjected allows helium gas to diffuse into their grain boundaries. Previous work has suggested that nano-heterophases, particularly 0D or 1D, might offer greater radiation resistance and helium tolerance, but experimental evidence of helium sequestration within the actual lattice of the nano-heterophase, rather than merely its phase boundary within the matrix, has been inconclusive.
In this work, the investigators used ab initio theoretical calculations along with a variety of experimental techniques, including scanning and transmission electron microscopy as well as high-energy X-ray diffraction studies at the 11-ID-C beamline of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to probe further and settle the question. The team also used grazing-incidence X-ray diffraction (GIRXD) at the Pohang Light Source.
After ab initio calculations to determine helium embedding energies of a variety of crystal structures and the X-ray diffraction patterns they would display with and without embedded helium, the research team synthesized model composite material samples combining an iron (Fe) matrix and a fayalite (Fe2SiO4) nano-heterophase for proof-of-principle demonstrations. After irradiation with helium ions, they were examined to determine the structural changes caused by helium ion implantation, including lattice distortions and the evolution of helium bubbles.
The computations indicated that given its helium embedding energy and atomic-scale free volume, Fe2SiO4 should be capable of storing helium in its lattice at a considerably lower energy cost than the Fe matrix. The GIXRD studies of samples both with and without Fe2SiO4 confirmed helium sequestration in the Fe2SiO4-containing samples, as indicated by definite differences in XRD peak intensities different from those seen with radiation damage. This is consistent with the predicted patterns from density functional theory calculations, including sequential broadening and sharpening of peaks along the diffraction angles in the Fe2SiO4-containing samples.
The findings all confirm that helium atoms are present within the Fe2SiO4 nano-heterophase lattice, basically forming a helide compound. Transmission electron microscopy (TEM) of the Fe2SiO4-containing samples also showed decreased size and number of helium bubbles, which indicates a higher resistance to helium damage and embrittlement in these helide-forming materials.
Based on this work demonstrating that nano-heterophases can absorb and store helium both at their phase boundaries and with their lattice, the investigators believe that perhaps as low as 1-2 volume percent of these helide-forming materials should be able to sequester most of the helium present within a fusion reactor, up to several thousand appm. They point out, however, that the capacity of different helide formers may vary, and that they may also attract other gaseous species such as hydrogen and tritium, which are produced from reactions caused by the fast neutrons resulting from the fusion reaction, thus cutting down the available free space for helium. The possible production of structural defects from neutron irradiation will also need to be further investigated.
Although it may not be quite as prominent an issue, the need for structural materials able to handle the literally star-like environment of a fusion reactor, including dealing with by-products of the reaction such as helium and tritium, is just as crucial for the success of fusion as a practical and sustainable energy source for humanity. This present work promises to go a long way toward finding and designing those necessary materials. – Mark Wolverton
See: S.Y. Kim1, S. Kavak2, K.G. Bayrak3, C. Sun4, H. Xu1, M.J. Lee5, D. Chen6, Y. Zhang7, E. Tekoglu1, D. Agaogullari2, E. Ayas3, E.S. Park5, J. Li1, “Demonstration of helide formation for fusion structural materials as natural lattice sinks for helium,” Acta Materialia, 266, 119654 (March 2024)
Author affiliations: 1Massachusetts Institute of Technology; 2Istanbul Technical University; 3Eskisehir Technical University; 4Clemson University; 5Seoul National University; 6University of Houston; 7Characterization.nano, Massachusetts Institute of Technology
This work was supported by Eni S.p.A. through the MIT Energy Initiative. S.Y.K. gratefully acknowledges partial financial support from the Kwanjeong Scholarship. M.J.L and E.S.P were supported by the Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) funded by the Korean Government (MSIT) (no. NRF-2019M3D1A1079215). The Texas Advanced Computing Center provided computing resources for the calculations. Microstructural characterization was performed in part in the MIT.nano Characterization Facilities. The authors acknowledge the support of the 5A-XRS beamline at the Pohang Light Source, Pohang Accelerator Laboratory, and the 11-ID-C beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH1135. The authors thank Ms. Ji Eun Lee for her assistance in GIXRD experiments, and Dr. Austin Akey, Dr. Yi-Sheng Chen, Dr. Ranming Niu, and Prof. Julie Cairney for their valuable discussions.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
