The original article by Deane Morrison was published in the University of Minnesota's Inquiry blog.
A team of University of Minnesota scientists, with critical information obtained through research at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory, has laid the groundwork for designing drugs to block the novel coronavirus from attaching itself to, and infecting, human cells. The work is published in the journal Nature.
The virus, SARS-CoV-2, is closely related to the SARS virus that caused devastation in 2002–2003. The team, led by University of Minnesota researcher Fang Li, studied how mutations that changed the structure of a SARS-CoV-2 protein enabled it to attach more securely to human cells than its predecessor.
“In general, by learning what structural features of viral proteins are most important in establishing contact with human cells, we can design drugs that seek them out and block their activity—like jamming their radar,” said Li, a professor in the Department of Veterinary and Biomedical Sciences, College of Veterinary Medicine.
Researchers from the Department of Biochemistry, Molecular Biology, and Biophysics, College of Biological Sciences, were also involved in the study. Li has been leading work on the SARS-CoV-2 virus since January.
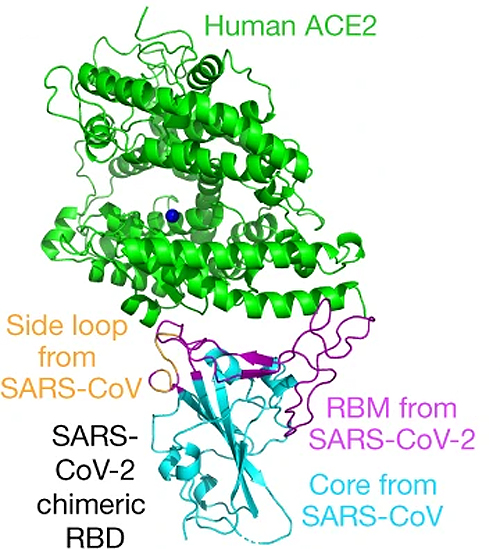
During infection, a “spike” protein on the surface of a SARS-CoV-2 particle attaches itself to a human “receptor” protein on the surfaces of human cells—notably lung cells. The receptor “receives” the virus, much as a lock receives a key.
By probing the features of the SARS-CoV-2 spike protein and the receptor with x-rays at the Northeastern Collaborative Access Team 24-ID-E beamline at the APS, Li and his colleagues discovered, for the first time, that just a few mutations had made a molecular “ridge” in the spike protein more compact than a similar structure in the 2002–2003 virus. This and other changes helped SARS-CoV-2 attach more strongly to the receptor, infect human cells better, and spread faster. The APS is an Office of Science user facility at Argonne.
“Our work can guide the development of monoclonal antibodies that would act like a drug to recognize and neutralize the receptor-binding part of the spike protein,” Li said. “Or, a part of the spike protein could become the basis of a vaccine. Overall, this study can guide structure-based intervention strategies that target receptor recognition by SARS-CoV-2. This is the focus of our current work.”
The researchers also discovered that a bat coronavirus recognizes the same human receptor as SARS-CoV-2, but the bat virus attaches to the human receptor rather poorly. However, a few mutations in the spike protein may have enhanced the ability of the bat virus to attach to the human receptor, leading to the bat virus jumping to humans and evolving to become SARS-CoV-2. Previous work by Li and others showed that one particular mutation allowed the 2002–2003 virus to get into human population; in the current work, it appeared that a number of mutations might be needed for SARS-CoV-2 to jump from bats to humans.
Also, two coronaviruses have been isolated from pangolins—a type of scaly, anteater-like mammal—in China. The researchers analyzed the structures of their spike proteins and found that one of the pangolin viruses could recognize the human receptor well, implying that pangolins might have helped the bat virus jump to humans by acting as intermediate hosts. (Note: No live coronaviruses are used in research at the APS.)
See: Jian Shang1,3, Gang Ye1,3, Ke Shi2,3, Yushun Wan1,3, Chuming Luo1, Hideki Aihara2, Qibin Geng1, Ashley Auerbach1, and Fang Li1, “Structural basis of receptor recognition by SARS-CoV-2,” Nature 581, 221 (14 May 2020). DOI: 10.1038/s41586-020-2179-y
Author affiliation: University of Minnesota
Correspondence: * [email protected]
This work was supported by National Institutes of Health (NIH) grants R01AI089728 and R01AI110700 (to F.L.) and R35GM118047 (to H.A.), The Northeastern Collaborative Access Team is funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Eiger 16M detector on the 24-ID-E beam line is funded by a NIH-ORIP HEI grant (S10OD021527). We thank staff at beamline 24-ID-E for assistance in data collection. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
