One challenge in the quest to improve battery performance is that the current technology of choice, lithium-ion batteries, can be flammable. Devices using aqueous electrolytes have the potential of operating more safely, but they face a different issue; increasing the concentration of salts should increase the conductivity of the electrolyte, but too high a concentration inhibits the flow of lithium, undermining that advantage.
Now researchers using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory have explained how the behavior of the electrolyte affects battery performance, paving the way for more efficient designs.
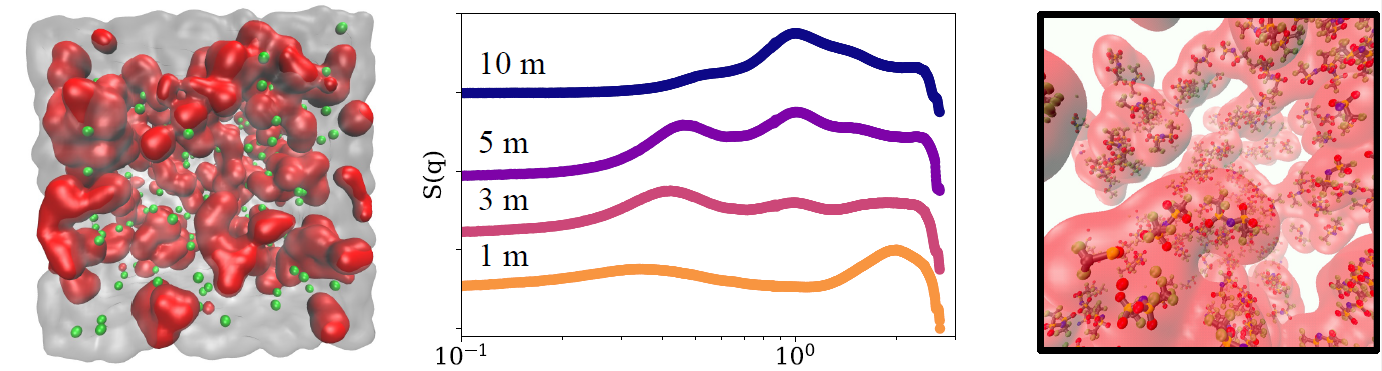
Previous studies had shown that an electrolyte consisting of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in water retained high conductivity even at very high concentrations of the salt. They also showed that the electrolyte had nanometer-scale areas where the anion was concentrated in long molecular chains and other regions that were mainly water. With such a structure, the lithium cations can move easily through the water-rich areas, increasing conductivity. To better understand what led to that structure, researchers turned to X-ray scattering methods.
To see how different solvents might affect the nanostructure, the team compared aqueous LiTFSI with the same salt dissolved in methanol and acetonitrile. They examined different molar concentrations of the salt, from 1 m to 10 m. They simultaneously collected two types of X-ray measurements, small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS), which allowed them to examine the electrolyte at different scales.
In the water, the anions arrange themselves into long, well-ordered chains. In the methanol, the same salt anions are somewhat ordered, but less so than in the water. In the acetonitrile, there is almost no chaining. The reason, the researchers found, is that water molecules provide two hydrogen-bonding sites. That allows one hydrogen atom in the water molecule to bond to an oxygen atom on one salt molecule, while the water’s second hydrogen bonds to another salt molecule, linking them together in a chain. In the methanol, the molecules have only a single hydrogen bonding site, so the solvent forms shorter chains with the salt. The acetonitrile provides no bonding sites, so chains do not form.
The team also performed molecular dynamics simulations of the systems to directly observe the electrolyte on the atomic scale. The SAXS and WAXS studies, in turn, served to validate that they were doing the simulations correctly.
The researchers performed their studies at beamline 12-ID-B at the APS. That beamline provides the ability to do SAXS and WAXS simultaneously, with WAXS providing measurements between 0.1 and 1 nm and SAXS covering the range from 1 to 100 nm. This was the first study of these materials to apply these X-ray scattering techniques. Previous measurements had been made with Raman, nuclear magnetic resonance, and infrared spectroscopies, but those only provide resolutions between 1 and 20 nm, a narrower range than the X-ray measurements.
The researchers would like to expand on this work by exploring different compositions of the electrolyte. For instance, they’re mixing water and methanol in different ratios to see how those affect the structure and transport properties of the material. There are also many salt options they can examine. Instead of lithium, they could use sodium, potassium, zinc, magnesium, calcium, or aluminum. Different combinations could provide different chain lengths and offer different properties. The team hopes to apply artificial intelligence to the measurements they collect so that a computer can predict which electrolyte recipe will provide the most desirable properties. – Neil Savage
See: L. Trojanowski1, X. Lyu2, S-C. Lee1, S. Seifert3, Y.Z.1, T. Li2,3, “Molecular origin of nanoscale anion ordering of LiTFSI electrolytes revealed through SAXS/WAXS and molecular dynamics simulations,” ACS Energy Lett. 10, 2, 696-702 (2025)
Author affiliations: 1University of Michigan, 2Northern Illinois University, 3Argonne National Laboratory.
This work was performed at the Advanced Photon Source and the Center for Nanoscale Materials of Argonne National Laboratory, U.S. Department of Energy Office of Science User Facilities, and was supported by the U.S. DOE, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. T. Li is thankful for the support from the U.S. National Science Foundation (Grant No. 2208972, 2120559, 2342334, and 2323117). Y Z acknowledges the support from the U.S. National Science Foundation (Grant No. 2323118). L. Trojanowski would like to thank Haimeng Wang for his assistance with the project.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
