Halide perovskite semiconductors have a formula of ABX3, in which A is a cation, B is typically lead, and X is iodine (I) or bromide (Br). By mixing the halides to incorporate both I and Br, researchers can tune the optical bandgap, a property that determines the wavelengths of light that the crystals absorb. Bromide-rich perovskites with a wide bandgap can operate at much higher voltages than conventional semiconductors such as silicon and have been extensively explored as light absorbers in solar cells, especially multijunction solar cells – devices in which light absorbers with complementary bandgaps are stacked on a single substrate to use the solar spectrum effectively and increase photovoltaic efficiency.
However, solution-processed wide-bandgap perovskite thin films are prone to forming a wrinkled morphology, a quality that prevents their use in multijunction solar cells where tens of ultrathin layers are laid on top of each other in complex stacks. Why and how these wrinkles form has been unclear. Using various analytical approaches, including X-ray fluorescence microscopy, a team of researchers helped answer these questions, providing insights that could lead to new protocols for synthesizing mixed-halide perovskite thin films that resist wrinkling.
The team used the resources of beamline 2-ID-D at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
The researchers worked with mixed-halide perovskite. They synthesized them with an antisolvent-based method, in which the perovskites crystallize out of solution after adding a solvent (ethyl acetate) in which they are not soluble. By independently changing the content of methylammonium (MA) and/or bromide (Br), the researchers altered the bandgap of the resulting material between 1.55 eV and 2.38 eV.
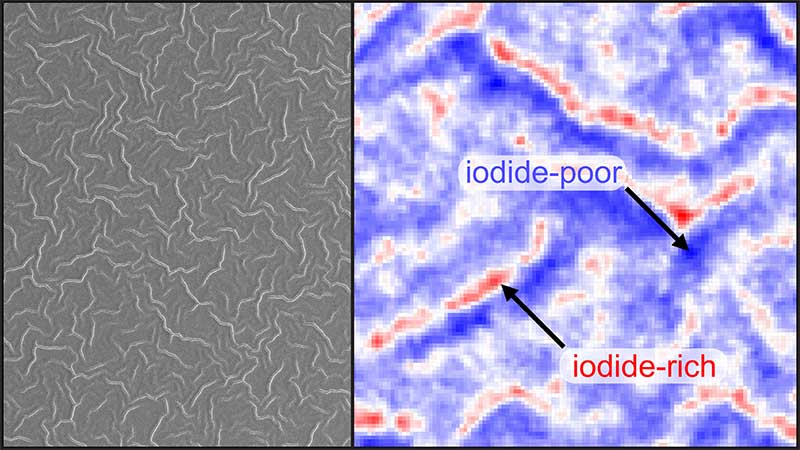
Electron microscopy showed that films with lower MA and Br contents had a smooth surface. However, those richer in MA and/or Br showed wrinkles on the surface with heights as large at 1.5-2 mm and width as large as 2-5 mm. Another look at the crystalline structure of different films using X-ray scattering showed that the smooth films were made of randomly oriented crystallites, but those with wrinkled surfaces were made of crystallites preferentially ordered in a single plane.
The research team next performed X-ray scattering measurements during film formation, comparing Br-heavy films that were either MA-poor (smooth) or MA-rich (wrinkled). The wrinkled film quickly developed crystals upon antisolvent addition, in contrast to the smooth film that crystallized more slowly. The authors say this indicates that Br-rich perovskite materials formed first in the films due to their low solubility in the precursor solution and encouraged by their chemical interactions with MA. This was followed by I-rich materials that crystallized later. This difference in crystallization rates of different perovskite phases is what leads to the formation of wrinkles.
The researchers then used nanoscopic X-ray fluorescence at the APS to study the impact of wrinkling and found a homogenous distribution of Br and I in smooth films. However, the distribution was heterogenous in the wrinkled films, with more I-based perovskites present in the peaks of the wrinkles and more Br-based perovskites present in the valleys. This uneven distribution significantly affected the optical properties of the films; another technique called hyperspectral photoluminescence imaging showed that the peaks had lower bandgaps compared to the higher bandgap in the valleys due to the heterogeneity in I and Br distribution.
Further experiments demonstrated that this heterogeneity in the wrinkled films also led to the formation of sub-bandgap electronic defects and accelerated light-induced segregation of I and Br ions in the films. These issues can negatively affect the efficiency and stability of these films when used in solar cells.
Together, the study authors say, these findings paint a picture of wrinkle formation in MA- and Br-rich mixed-halide perovskite thin films that sets in within seconds of introducing the antisolvent. The differing crystallization rates of Br and I lead to mechanical stresses that cause the formation of I-rich peaks and Br-rich valleys, resulting in a heterogenous bandgap distribution across the film that ultimately harms solar cell performance. By adjusting various factors that affect crystallization kinetics, they add, researchers may eventually be able to produce mixed-halide perovskite films that resist wrinkling, improving solar cell efficiencies and enabling high-efficiency multijunction devices. – Christy Brownlee
See: K. Datta1,2, S.C.W van Laar1, M. Taddei3, J. Hidalgo2, T. Kodalle4, G.J.W. Aalbers1, B. Lai5, R.Li6, N. Tamura4, J.T.W. Frencken1, S.V.Q. Monnens1, R.J.E. Westbrook3, D.J. Graham3, C.M. Sutter-Fella4, J-P. Correa-Baena2, D.S. Ginger3,7, M.M. Wienk1, R.A.J. Janssen1,8, “Local halide heterogeneity drives surface wrinkling in mixed-halide wide-bandgap perovskites,” Nat Commun 16 1967 (2025)
Author affiliations: 1Eindhoven Institute of Technology; 2Georgia Institute of Technology; 3University of Washington; 4Lawrence Berkeley National Laboratory; 5Argonne National Laboratory; 6Brookhaven National Laboratory; 7Pacific Northwest National Laboratory; 8Dutch Institute of Fundamental Energy Research
R.A.J.J. acknowledges the Ministry of Education, Culture, and Science (Gravity program 024.001.035), the Netherlands Organization for Scientific Research (Joint Solar Programme III Project 680.91.011 and Spinoza grant) and the European Research Council (ERC) under the European Union’s Horizon Europe research and innovation programme (Grant Agreement No. 101098168, PERSTACK) for funding. M.T. acknowledges the Office of Naval Research (Award # N00014-20-1-2587) for funding. TOF-SIMS was carried out at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington which is supported in part by the National Science Foundation (awards NNCI-2025489, NNCI-1542101), the Molecular Engineering and Sciences Institute, and the Clean Energy Institute. The research used the CMS 11-BM beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office Science User Facility operated for DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. The research used beamline 12.3.2, a resource from the Advanced Light Source, a DOE Office of Science User Facility under Contract No. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. T.K. acknowledges support by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under Contract No. DE-AC02-05-CH11231 (D2S2 program KCD2S2). This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
