 Work to develop a vaccine to protect against human immunodeficiency virus (HIV) has been underway for four decades but we still have no effective vaccine. Treatments have been developed that allow people infected with HIV to live long and healthy lives, but a vaccine could prevent new infections, affecting about 1.3 million people annually worldwide, and could potentially eradicate the disease.
Work to develop a vaccine to protect against human immunodeficiency virus (HIV) has been underway for four decades but we still have no effective vaccine. Treatments have been developed that allow people infected with HIV to live long and healthy lives, but a vaccine could prevent new infections, affecting about 1.3 million people annually worldwide, and could potentially eradicate the disease.
Unfortunately, vaccine development has been plagued by challenges related to the evasive tactics of the virus, which mutates very quickly, and difficulties in obtaining a structure for the main envelope protein of the virus, Env, which was finally solved by electron microscopy and crystallography in 2013. HIV vaccine researchers have come to the conclusion that ideal vaccine immunogens designed to generate a protective immune response should elicit antibodies that can recognize HIV via a number of target sites on the Env protein. Broadly neutralizing antibodies to each of these sites have been identified in some people infected with HIV-1 and in some animal models but efforts to elicit this broad antibody recognition of the diverse HIV strains and subtypes in response to a vaccine have not been successful.
A recent study from a group at the Ragon Institute, Scripps Research Institute, Leipzig University, La Jolla Institute for Immunology, UC San Diego, Moderna, and Massachusetts Institute of Technology provides insights into antibody-based HIV vaccine development that could lead to the identification of vaccine immunogen candidates to elicit such broadly neutralizing antibodies.
Scripps Research investigators used resources of the National Institute of General Medical Science and National Cancer Institute Structural Biology Facility (GM/CA) at beamline 23-ID-B of the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
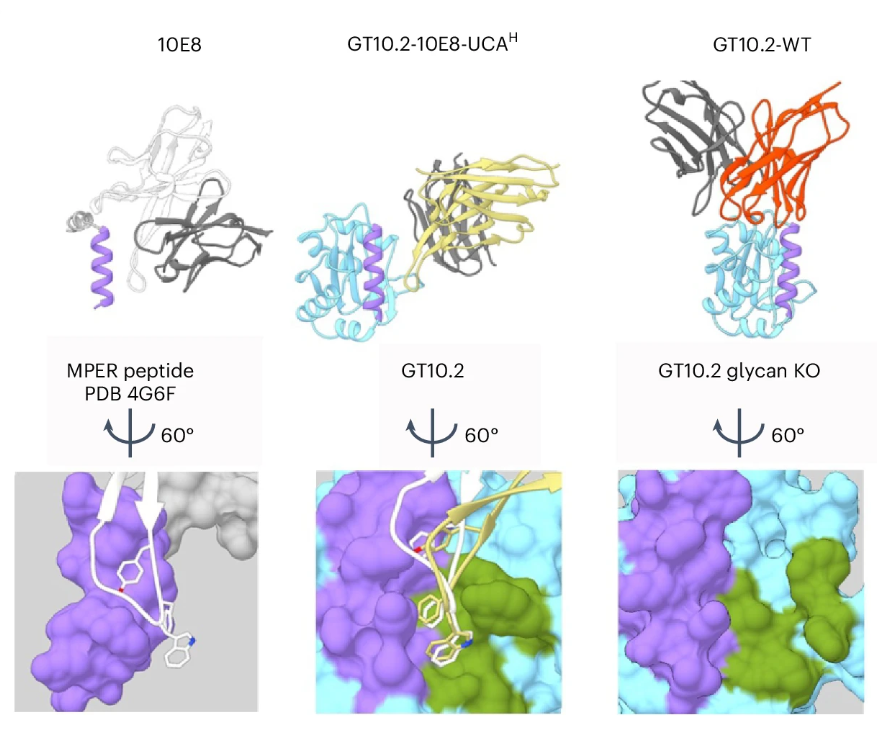
The research started with the observation that a broadly neutralizing antibody called 10E8 has excellent HIV neutralization properties but does not recognize self-antigens. This is an undesirable feature, as earlier antibodies to this membrane-proximal external region (MPER) site did. During an immune response that leads to the development of antibodies, precursor B cells that make a certain type of antibody are activated by the antigen they recognize (e.g., a viral protein), undergo mutations that increase their affinity for the antigen, and then start expanding to produce more of these cells.
With 10E8, the researchers identified precursors of the antibody, before these affinity mutations, and engineered immunogens to specifically activate these rare 10E8-class precursor B cells. This technique is called germline targeting.
To test their new immunogens, the researchers created mice that express the 10E8-class precursor B cells, which are normally only found in humans. They found that these B cells were functioning normally in mice, but when they immunized the mice with an immunogen designed to activate their targeted 10E8-class precursors B cells, they didn’t see the expansion they had hoped for, even though they could show that the B cells had high enough affinity to be activated.
They next tried to improve the effect of the immunogen with a technique to show more copies to the immune system and with another technique to deliver it in a formulation that would be more immunogenic but, although the formulation provided better expansion, it was still not what they were hoping for. Further experiments suggested that, although the B cells they wanted to expand had a high enough affinity for the desired antigens to be activated, they were being competed out by higher affinity B cells from the mouse immune system.
Crystal structures of the original 10E8 antibody and one of the high-affinity mouse antibodies, each in complex with 10E8 germline targeting immunogens, showed that the antibodies were binding to overlapping sites, explaining why the mouse antibodies were able to out-compete 10E8-class B cells.
To overcome this problem, the team further improved their immunogen to obtain higher affinity for the precursor B cell antibodies than the mouse competitors. A slight boost in affinity was enough to overcome the gap in affinity with the mouse B cells and allowed for the expansion of the 10E8-class precursor B cells that they were looking for. The team also tested their immunogen against several slightly different yet related 10E8-class precursors.
When they introduced a 10E8-class precursor B cell with higher affinity than the previously tested B cell into the mouse model, the expansion of 10E8-class precursors improved even further, clearly demonstrating the power of their system to develop and optimize immunogen/B cell combinations for vaccine development. – Sandy Field
See: R. Ray1, T. Schiffner2,3, X. Wang1, Y. Yan1, K. Rantalainen2, C-C.D. Lee2, S. Parikh1, R.A. Reyes1, G.A. Dale1, Y-C. Lin1, Z. Zhang1, S. Pecetta1, S. Giguere1, O. Swanson2, S. Kratochvil1, E. Melzi1, I. Phung2,4, L. Madungwe1, O. Kalyuzhniy2, J. Warner1, S.R. Weldon1, R. Tingle2, E. Lamperti1, K.H. Kirsch1, N. Phelps2, E. Georgeson2, Y. Adachi2, M. Kubitz2, U. Nair1, S. Crotty2,4,5, I.A. Wilson2, W.R. Schief1,2,6, F.D. Batista1,7, “Affinity gaps among B cells in germinal centers drive the selection of MPER precursors,” Nat Immunol 25 1083-1096 (2024).
Author affiliations: 1Ragon Institute of Mass General, MIT and Harvard; 2Scripps Research Institute; 3Leipzig University Medical Faculty; 4La Jolla Institute for Immunology; 5University of California San Diego; 6Moderna Inc.; 7Massachusetts Institute of Technology.
We thank all members of the laboratory of F.D.B. for their assistance with the immunization experiments. Support for this work was provided by the Collaboration for AIDS Vaccine Discovery grants OPP1084519 and OPP1147787 (to W.R.S.), INV-034657 (to W.R.S. and I.A.W.), OPP1196345/INV-008813 (to W.R.S.) and INV009585 and INV046626 (to F.D.B.) funded by the Bill and Melinda Gates Foundation. Funding was also provided by the National Institute of Allergy and Infectious Diseases UM1 AI144462 (Scripps Consortium for HIV/AIDS Vaccine Development; to W.R.S., I.A.W. and F.D.B.) and R01 AI147826 (to W.R.S., I.A.W. and F.D.B.) and by the Ragon Institute of Mass General, MIT and Harvard (to W.R.S. and F.D.B.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
