Sugar molecules in our bodies, derived primarily from food, can spontaneously adhere to various proteins, a process called glycation. Glycation can form dangerous Advanced Glycation End Products (AGEs) that lead to various pathologies like Alzheimer’s disease and diabetes, but it can also disable proteins that help cancer cells proliferate. In the early 2000s, scientists discovered that an enzyme called fructosamine-3-Kinase (FN3K) reverses protein glycation. That has made FN3K a valuable target for drug developers hoping to control when and where glycation occurs.
The data needed for such work has been lacking. But a new study published in Nature Communications involving high-resolution structures determined from data collected at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, reveals how FN3K deglycates a protein. These findings can serve as the basis for structure-based and in silico drug design targeting FN3K.
Glycation normally occurs in the bloodstream, but it can happen quickly and spontaneously wherever sugar levels are high. One place would be a tumor microenvironment, where cancer cells require the energy from sugar to proliferate.
Proliferation of cancer cells is also aided by a protein called NRF2 (nuclear factor erythroid 2-related factor 2). This transcription factor regulates genes involved in cell growth and survival. It can both suppress and promote tumors, depending on the type of cancer and what stage it’s in. Early on, it’s thought to suppress tumors; later, it’s thought to promote them.
When NRF2 is glycated, it loses its stability and is rendered ineffective at protecting cancer cells. But NRF2 can regain this detrimental function when the sugar is removed—deglycated—by the enzyme FN3K, according to a 2019 study. This study opened two new ways of thinking about how to limit cancer cell proliferation: targeting NRF2 directly or modulating its activity through FN3K—a back door approach that would prevent the enzyme from deglycating NRF2.
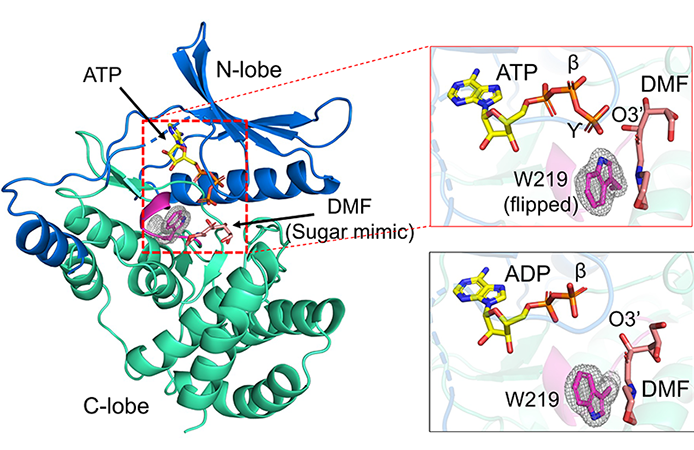
With the 2019 study in mind, the scientists behind the current research set out to explore the therapeutic potential of FN3K. They determined a series of crystal structures of human FN3K (HsFN3K) in its unbound, or apo, form. Some variation of a deglycating enzyme occurs in nearly every form of life; the human form is the only one to feature the amino acid tryptophan near its core catalytic site.
The scientists also determined crystal structures of HsFN3K bound to an analog of glycated NRF2 and the nucleotide ATP triggering different catalytic states—ATP prior to phosphorylation, ADP following phosphorylation, and AMPPNP, an ATP analog, for comparison. The X-ray diffraction data were collected at the Northeast Collaborative Access Team (NE-CAT) beamline at 24-ID-E of the APS.
With careful scrutiny of such high-resolution structures, the team deciphered what no one had seen before. In the pre-catalytic state, the tryptophan recognized the ATP, then flipped 180 degrees. That caused a conformational change in the sugar moiety on the NRF2 analog that made it receptive to phosphorylation; addition of the phosphate destabilized the sugar half and removed it from the protein. Had the resolution been any lower, scientists would not have perceived and recognized the importance of the tryptophan flip. Analyzing their structures, they found that it did not occur with ADP or AMPPNP; it only happened with ATP.
What about tryptophan? To investigate its role, the team substituted a different amino acid to see if deglycation would still take place. It didn’t, leading the team to hypothesize that tryptophan may function as an ATP sensor, promoting HsFN3K’s kinase activity, even though it’s not part of the usual kinase classical active site players. But one tryptophan substitution gave the opposite result—changing it to a histidine, present in several versions of this enzyme from other species, made HsFN3K unusually hyperactive.
These findings have broad implications for advancing scientific knowledge and conducting basic research. The scientists hypothesize that in humans, evolution enhanced cellular homeostasis by slowing down HsFN3K’s glycating activity through tryptophan, creating an advantageous baseline level of glycation.
As for basic research, many scientists use a derivative of ATP that doesn’t trigger phosphorylation (AMPPNP) to study how ATP interacts with proteins without bothering with catalytic reaction. Only one atom distinguishes ATP from AMPPNP, but that one atom makes all the difference in the world, according to the authors of this study. They found that AMPPNP sits slightly differently from ATP in HsFN3K, preventing the tryptophan from flipping. While they don’t believe that their findings invalidate studies using AMPPNP, they do believe that in some cases, scientists should be careful how they interpret findings.
When it comes to drug development, kinases like FN3K are much easier to target than transcription factors like NRF2. However, there are many kinases present in the cell, so drug developers must be very specific when targeting one in particular. Because tryptophan is very specific for HsFN3K, pharmaceutical companies may try inventing compounds that prevent the tryptophan from flipping and triggering deglycation.
As for next steps, the team may look more carefully at conditions that regulate the interactions between FN3K and select regions of NRF2, as well as other enzymes with different substrate requirements. – Judy Myers
See: A. Garg1, K.F. On1, Y. Xiao2, E. Elkayam1, P. Cifani1, Y. David2, L. Joshua-Tor1, “The molecular basis of human FN3K mediated phosphorylation of glycated substrates,” Nat Commun 16 941 (2025)
Author affiliations: 1Cold Spring Harbor Laboratory; 2Memorial Sloan Kettering Cancer Center.
We thank Hans-Guido Wendel and members of Joshua-Tor laboratory for valuable discussions, and the CSHL core-proteomics facility for support with mass-spectrometry analysis. We thank the support of the beamline staff at National Synchrotron Light Source II (AMX-17-ID-1), a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. We highly appreciate the support of the beamline staff at Northeastern Collaborative Access Team (NE-CAT) beamline 24-ID-E, funded by NIH grant GM P30G124165 and operated for DOE Office of Science by Argonne National Laboratories under contract DE-AC0206CH11357, for help with X-ray data collection. This work was supported by STARR Grant #36210201 (to Y.D. and L.J.). The mass spectrometry Shared Resource was supported by the CSHL Cancer Center Support Grant #5P30CA045508. L.J. is an investigator of the Howards Hughes Medical Institute.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
