The IDH1 protein (isocitrate dehydrogenase 1) helps turn the food we eat into chemical energy that powers our cells. However, an alteration in just one of the protein’s amino acids has also been linked to brain and blood cancer. Years ago, scientists developed compounds that inhibited the mutant but not the normal (wild type) protein. That was good, but no one knew how the compounds worked.
Recent research published in Nature Communications describes a newly designed compound that binds to IDH1, away from the active site, changing the protein’s shape so it traps the mutant in an inactive form while allowing the normal (wild type) protein to morph between multiple active conformations. Data collected at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, as well as from nuclear magnetic resonance (NMR) spectroscopy measurements, lay the foundation for further investigations in anticancer drug development and basic research.
IDH1 is the third enzyme in the Krebs cycle, an eight-step process kicked off by a molecule derived from sugar, fat, or protein. The first molecule is transformed into a second molecule, which is transformed into a third molecule, and so on, each new molecule (called an intermediate) serving as the raw material for the next step in the cycle. The final products of the cycle are ATP, the energy source for cells; NADPH, which powers further production of ATP; and compounds used to synthesize amino acids.
Within the Krebs cycle, IDH1 transforms isocitrate into aKG (alpha-ketoglutarate), which regulates gene expression. Mutant IDH1, however, turns aKG into 2-HG (2-hydroxyglutarate), affecting cell metabolism and dysregulating gene expression. 2-HG is found at high levels in glioma, glioblastoma, and acute myeloid leukemia (AML).
Because wild type IDH1 is critical to cell survival, anti-cancer drug developers sought to design a molecule that could block the mutant but not the wild type—an extremely difficult task when only one amino acid separated the two. In fact, the FDA approved such a therapeutic, called ivosidenib, in 2018, but atomic resolution details on how it worked remained a mystery.
To help accelerate the discovery of selective inhibitors, a team of scientists at Merck & Co. initiated a program that included structural biology, biophysics, NMR, mass spectrometry, and biochemical assays. The team was able to better understand the mechanism of action for their screening hits, and later, an approved drug by combining insights from different methods.
The team first combed through Merck’s compound library to identify candidate molecules that prevented the mutant from producing 2HG but did not affect the activity of wild type IDH1. They got a hit and called it compound 1.
Mass spectrometry and surface plasmon resonance (SPR) confirmed that compound 1 bound to both the mutant and wild type IDH1. In many cases, drug compounds inactivate enzymes by occupying their active site, preventing the activating molecule from binding. Sometimes, however, drug compounds bind to a different site—called allosteric—so binding doesn’t necessarily translate to inactivation. Through mass spectrometry binding studies, the scientists also found that substrates isocitrate and aKG, respectively, displaced compound 1 from wild type but not mutant IDH1.
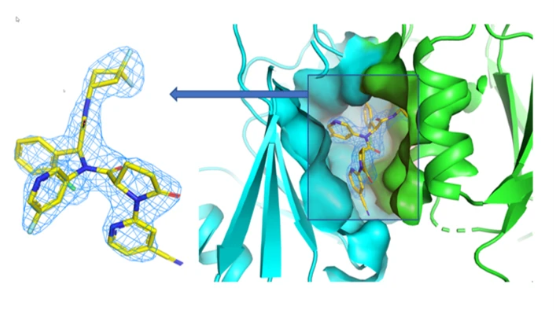
Enabled by X-ray crystallography at the Industrial Macromolecular Crystallography Association Collaborative Access Team (IMCA-CAT) beamline at 17-ID of the APS, the scientists next determined the atomic structure of compound 1 in complex with wild type and mutant IDH1, as well as IDH1 in complex with optimized versions of compound 1 and the FDA-approved ivosidenib.
NMR was used to study changes to cofactors, substrates, and products due to reaction with wild-type and mutant enzymes. Additionally, NMR binding studies revealed that when compound 1 bound to wild type IDH1, it flopped between multiple conformations, but the mutant was trapped into a single open inactive state. Here was the mechanism of action.
They made three significant discoveries: they identified for the first time, the ivosidenib binding site and found that compound 1 binds to mutant IDH1 differently; compound 1 binds to both mutant and wild type IDH1 in an allosteric site distant from the mutation without directly involving the mutation; and they found motional differences in compound 1 binding to wild-type and mutant IDH1.
The Merck team’s insights can now be applied to the potential development of an anti-cancer therapeutic against brain cancer and AML. Additionally, they believe there’s a bigger story behind IDH1 and its unusual allosteric binding mechanism, which basic research scientists may now wish to pursue. – Judy Myers.
See: M.A. McCoy1, J. Lu1, F.R. Miller1, S.M. Soisson1, M.H. Lam1, C. Fischer1, “Biostructural, biochemical and biophysical studies of mutant IDH1,” Nat Commun 15, 7877 (Sept. 2024)
Author affiliations: 1Merck &Co. Inc.
The authors wish to acknowledge the assistance with experiments (Brian Lacey, Peter Spacciapoli, Anthony Donofrio, Xiaohua Huang, Gopal Parthasarathy, Pravien Abeywickrema) and discussions of the results (Astrid Kral, John Lampe, Elliott Nickbarg, Daniel Klein, Christine Andrews) described in the publication.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
