Dropped your phone recently? One possible reason it didn't break is that it's made from metallic glass. Metallic glasses are highly disordered at the atomic level but are macroscopically strong and resistant to wear and corrosion. Because of their unique chemical structure, however, they deform easily under external stress.
Recently, researchers from German and U.S. universities and institutions have investigated how metallic glass responds to external stress to better understand its pertinent physical changes as well as determine whether stress-induced deformation - like a ding on a dropped cell phone - could be reversible. The researchers observed that even small amounts of pressure will produce highly accelerated rates of movement of atoms within the material (the first direct experimental evidence for low-barrier transport) and that the structural atomic changes which support this atomic-level transport can also explain behaviors involving reversing deformation.
For their investigation, the team used a type of metallic glass known as Vitreloy 105, which is an alloy of zirconium, titanium, copper, nickel and aluminum. At a macroscopic level, the material can withstand pressures in the range of gigapascals (the strength required by aircraft metals). Microscopically, the highly disordered structure of metallic glasses means that the atoms within exist at a broad range of energy levels.
Mathematical simulations support a two-component hypothesis, meaning that metallic glasses under stress order atomically into two distinct components. One component is elastic (which returns to its original shape after stress is removed), stiff, and stable. The second is plastic (which remains in a new shape after stress is removed) at the microscopic level and allows for the transport of atoms. If the plastic component of the material does not have a true elastic limit¾a stress beyond which its deformations become irreversible¾then the elastic limit for the material is that of the macroscopic yield stress, or the pressure at which the material's macroscopic deformation changes from elastic to plastic. And if the plastic component does not have a true elastic limit, then it might be possible to rejuvenate the material to improve its plasticity after deformation.
To observe the behavior of the zirconium-based metallic glass under stress and test the two-component hypothesis, the team took X-ray photon correlation spectroscopy using at the 8-ID-E beam line at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory. This allowed the team to determine how long it took for the material to return to equilibrium (the relaxation time) as a function of both the stress applied and the time after application of stress.
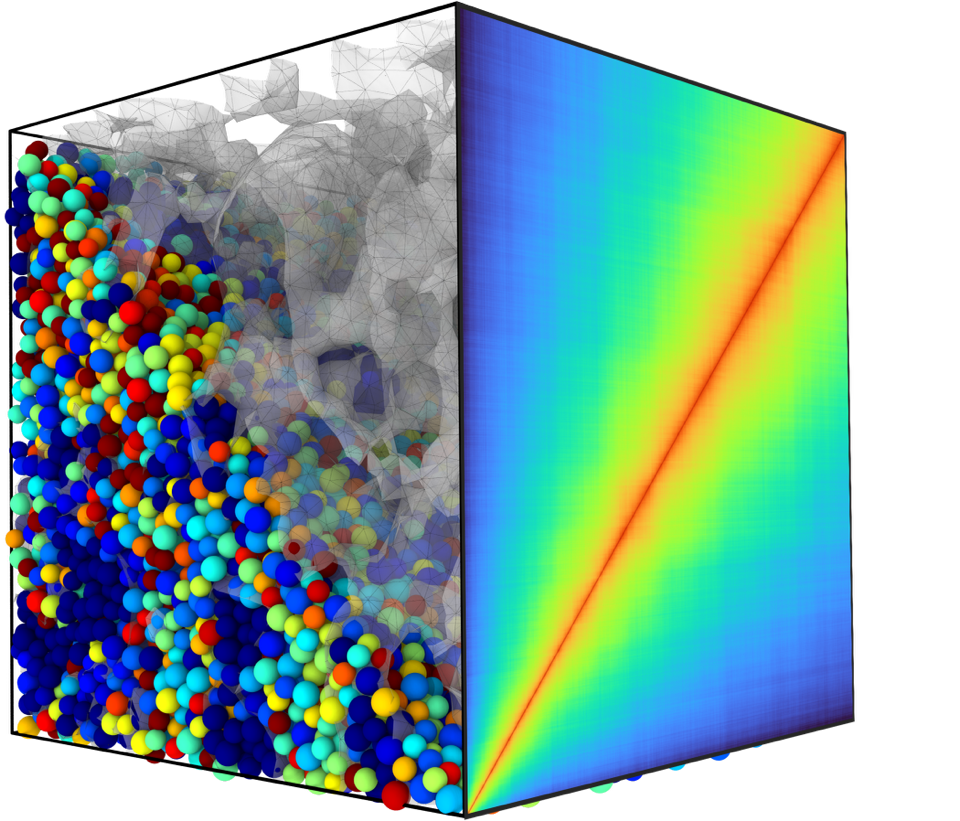
Figure 1 shows two representations of the metallic glass under stress. The team found that even a small amount of stress decreased the relaxation times by an order of magnitude. The team determined this change in relaxation times was a product of the plastic component of the material. The team also found that the values for the relaxation times decreased as a function of time after the stress was removed; they explained this as a result of the exhaustion of possible structural atomic reorganizations within the plastic component. Additionally, the team found that the distribution of relaxation times changed from a compressed form to an exponential form.
In summary, the team found three main categories of response: no transport without applied stress; highly-accelerated transport in response to stress which tapers off to behave like the no-stress situation; and immediate cessation of transport on removal of applied stress, also tapering off to behave like the no-stress situation.
From the changes to the relaxation times under external stress, the team concluded that the two-component hypothesis for zirconium-based metallic glass is accurate. They further concluded that the elastic, stiff, and stable component is responsible for the high macroscopic yield strength of the metallic glass, while the plastic component is responsible for the highly-accelerated transport of atoms and the decrease in relaxation times. Based on the extremely low stress required to induce transport, the team concluded that microscopic structural deformation within a zirconium-based metallic glass should be recoverable, which is good news for all of us with dropped phones. – Mary Agner
See: B. Riechers1, A. Das2, R. Rashidi1,3, E. Dufresne4, R. Maaß1,3,5, “Metallic glasses: Elastically stiff yet flowing at any stress,” Mat Today 82 92-98 (2025)
Author affiliations: 1Federal Institute of Materials Research and Testing (BAM); 2Cornell University; 3Technical University of Munich; 4Argonne National Laboratory; 5University of Illinois Urbana-Champaign.
The authors thank P.M. Derlet and Mo Li for fruitful discussions. Financial support by the European Union’s Horizon Europe Framework Programme (HORIZON) under the Marie Skłodowska Curie grant agreement (No. 101063523), the German Research Foundation, DFG, (Grant No. 466247278), and the Adolf Martens Fond of BAM are gratefully acknowledged. A.D. acknowledges the support of the National Science Foundation (BIO, ENG and MPS Directorates) under award DMR-1829070 and DMR-2342336 as well as the support of the Air Force Research Laboratory under agreement number FA8650-22-2-5200. The XPCS experiments were performed at the X-ray Science Division beamline 8ID-E of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
