A recently discovered superconducting compound, the nickel-oxide Nd0.8Sr0.2NiO2, is composed of the elements neodymium (Nd), strontium (Sr), nickel (Ni), and oxygen (O). Scientists produce this compound through a process called topotactic reduction, which transforms the relatively stable parent compound into a “high-temperature” superconducting form. These two compounds are nearly identical, except that the superconducting form has fewer oxygen atoms than the parent compound.
Unfortunately, the topotactic reduction process is extremely difficult to perform, and oftentimes the end-product will not superconduct. To address this issue a group of researchers recently studied the synthesis of superconducting Nd0.8Sr0.2NiO2 using synchrotron X-ray scattering and absorption near-edge spectroscopy (XANES). These X-ray observations of the real-time reduction process were gathered at beamlines 12-ID-D, 20-BM-B, 25-ID-C, 33-BM-C, and 33-ID-D of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
The experimental results demonstrate that successful synthesis depends upon the formation of an initial ultra-thin layer at the bottom surface of the originating parent compound. Failure to properly form and maintain this layer during topotactic reduction results in a non-superconducting product. The insights gained through the X-ray measurements and other experimental methods will allow scientists to reliably synthesize superconducting Nd0.8Sr0.2NiO2, spurring future investigations into its properties, and may even provide new information about the general mechanisms driving high-temperature superconductivity.
The first high-temperature superconductor was discovered in 1986, in a copper-oxide compound. Scientists immediately theorized that other oxides, including certain nickel oxides, should also exhibit this property. In 2019 researchers finally detected high-temperature superconductivity in a nickel-oxide compound, namely the one examined for this study. Researchers set out to enhance the successful synthesis of superconducting Nd0.8Sr0.2NiO2 by observing in minute detail each phase of the topotactic reduction process.
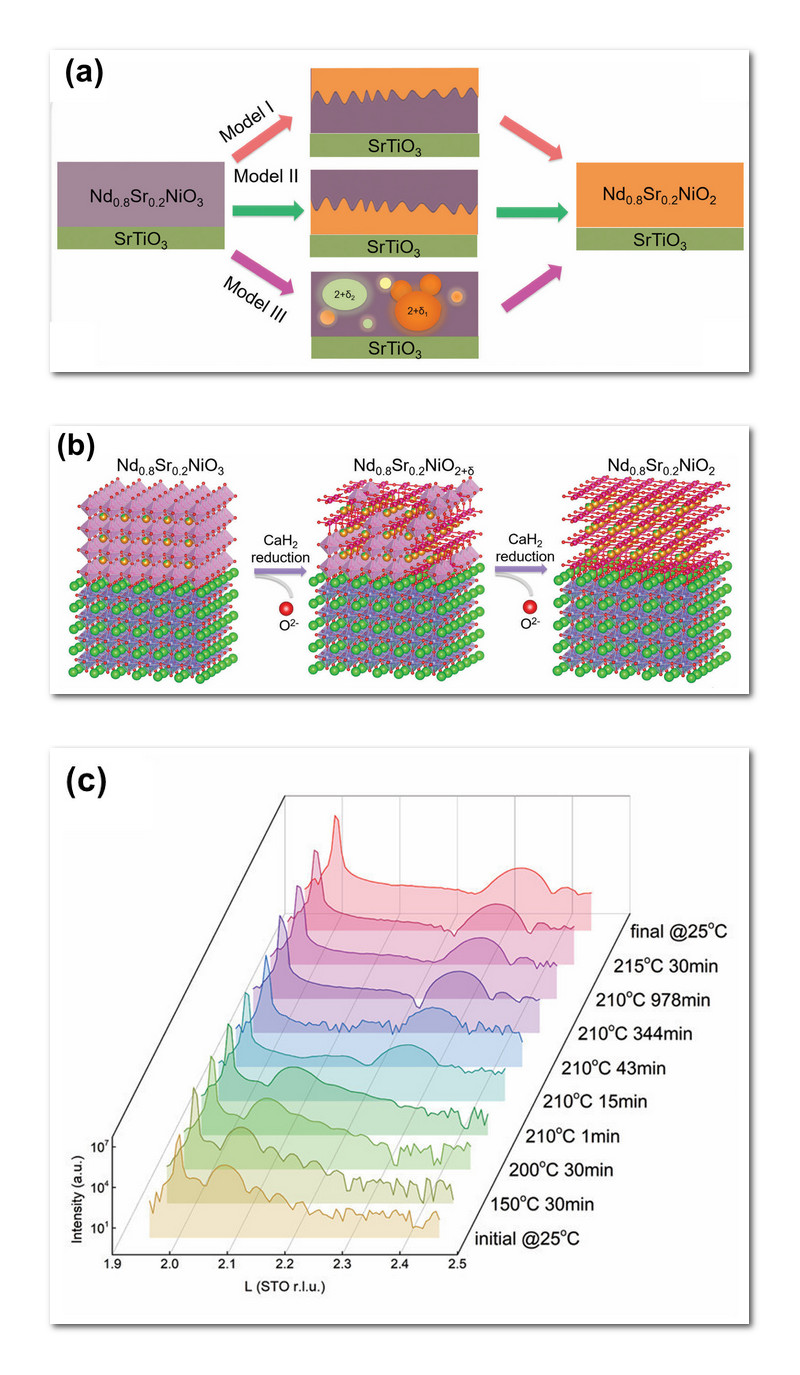
Experimental samples were created by vapor deposition on a substrate of strontium-titanium-oxide, yielding a thin-film system (left-side of Fig 1a). Topotactic reduction then gradually removed oxygen atoms from the thin-film samples, forming superconducting Nd0.8Sr0.2NiO2 (far-right of Fig. 1a).
When an oxide loses oxygen via a chemical process it is said to be reduced. For instance, the reduction of rust (iron oxide) through a chemical reaction changes the oxidation state of iron, separating it from the oxygen. Similarly, topotactic reduction transforms the parent compound by removing oxygen to form superconducting Nd0.8Sr0.2NiO2, while also retaining the parent's overall crystalline structure in the final superconducting form.
Fig. 1b illustrates the topotactic reduction process. The 11-nanometer-thick parent compound appears on the left side of Fig. 1b, exhibiting a three-dimensional perovskite structure in which oxygen atoms form little diamond-like shapes, with nickel at the center and strontium atoms in between. When heated with calcium hydride (CaH2), oxygen gradually leaves the compound, leading to an intermediate compound (middle), and finally the desired end product (far-right Fig. 1b) that consists of two-dimensional sheets forming an “infinite-layer nickelate”. This basically means that topotactic reduction continuously converts the 3D parent compound into stacks of 2D sheets. These sheets retain the “square-planar” crystalline structure of the original parent compound that is essential for superconductivity in the nickel oxides. X-ray scattering intensities are displayed in Fig. 1c, alongside the corresponding durations and temperatures employed during topotactic reduction.
The researchers considered three possible reduction pathways for the topotactic process (middle panel of Fig. 1a). In Model I, hydrogen diffuses into the uppermost surface, reducing the parent compound in a top-down process. In Model II, hydrogen first starts reducing the parent compound at the substrate interface, in a bottom-up process. Finally, in Model III, hydrogen initiates reduction throughout the parent compound at random nucleation sites.
The experimental measurements identified Model II as the reduction pathway. Hydrogen liberated from CaH2 initially reduces the parent compound at the SrTiO3 interface, forming an extremely thin layer there. From this initially-reduced layer, new 2D layers of nickel oxide consecutively form and grow to a thickness around nine nanometers (9 nm). Atop the combined 2D superconducting layers was a much thinner (1.6 nm) non-superconducting layer, which helped remove any residual hydrogen from the 2D layers, demonstrating that hydrogen is not necessary to achieve high-temperature superconductivity in this compound.
In summary, the research results reveal key details of the processes required to successfully form superconducting Nd0.8Sr0.2NiO2. Looking forward, the researchers envision enhancing and stabilizing these and other thin-film superconductors by combining them with materials similar to the substrate. – Philip Koth
See: Y. Li1, C. Liu1, H. Zheng1, J.S. Liang1, Z. Zhu2, X. Yan1, H. Cao1, K.V.L.V. Narayanachari3, B. Paudel2, K.P. Koriala2, Z. Zhang1, B. Fisher1, H. Wang4, E. Karapetrova1, C. Sun1, S. Kelly1, D. Phelan1, Y. Du2, B. Buchholz3, J.F. Mitchell1, A. Bhattacharya1, D.D. Fong1, H. Zhou1, “On the topotactic phase transition achieving superconducting infinite-layer nickelates,” Adv. Materials 36 40 2402484 (2024)
Author affiliations: 1Argonne National Laboratory; 2Pacific Northwest National Laboratory; 3Northwestern University; 4Institute of High-Energy Physics, Chinese Academy of Sciences
The research was supported by U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. The research was performed at beamlines 33-BM-C, 33-ID-E, 12-ID-D, 20-BM-B, and 25-ID of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Materials growth made use of the Pulsed Laser Deposition Shared Facility at the Materials Research Center at Northwestern University supported by the National Science Foundation MRSEC program (DMR-1720139) and the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633). SIMS measurement and data analysis were supported by the DOE BES Division of Materials Sciences and Engineering under Award No. 10122 via a project award (Award DOI: 10.46936/cpcy.proj.2021.60271/60008423) from the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Biological and Environmental Research program under Contract No. DE-AC05-76RL01830. C.L. acknowledges partial financial support from the College of Arts and Sciences, University at Buffalo, SUNY.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
