The growth of electric vehicles continues to drive demand for rechargeable lithium-ion batteries, and researchers are working to improve such batteries by increasing their energy density and lengthening their lifespan. One of the factors researchers must deal with is self-discharge, in which batteries lose stored energy even when not in use.
Now scientists have discovered what appears to be the primary mechanism for self-discharge from high voltages. The research team used the resources of the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
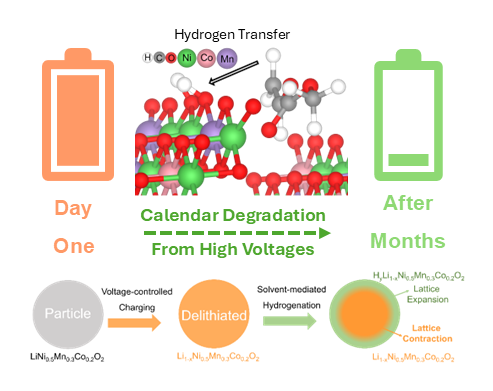
One prevailing model on the cause of the self-discharge was lithium ions diffusing into the cathode from the electrolyte, the mass of lithium salts dissolved in carbonate solvents that transports ions between the electrodes. On the other hand, theoretical predictions suggested that the electrolyte can decompose on the reactive surface of the cathode. Researchers discovered that in fact the hydrogen atoms coming from the carbonate solvents can insert themselves into the cathode, a process called hydrogenation, causing self-discharge.
The researchers combined electrochemical studies of cathodes with thermodynamic and computational analysis and synchrotron X-ray studies, using both model thin films and commercial cathode materials made of nickel-cobalt-manganese oxide. The X-rays revealed that the hydrogen had attached to the nickel in the cathode, with only minor changes to the cobalt and negligible changes to the manganese. They knew the source was the electrolyte, because they had replaced ordinary hydrogen in the electrolyte with deuterium, a hydrogen isotope, and that was what they saw in the cathode.
Researchers also found that hydrogenation was more pronounced at the surface of the material, rather than in the bulk. That explains another issue with batteries; not only does self-discharge mean that the amount of stored energy decreases over time, it also leads to uneven distributions of the elements within cathodes, shortening the battery’s lifetime. That’s because adding hydrogen atoms to the delithiated cathodes causes the crystal lattice of the surface to expand while the lattice of the bulk remains the same, causing strain between the layers that leads to cracks.
Hydrogen atoms do not return to the solvent’s molecular structure when the battery is recharged. Instead, protons can trigger side reactions, causing corrosion within the battery or creating hydrogen gas, further damaging the devices.
The team performed hard X-ray absorption spectroscopy (XAS) in the grazing incidence geometry at APS beamline 20-ID-B to see the surface and bulk state of the nickel in the thin films. They also used surface diffraction at beamline 33-ID-D to reveal the structure changes of the self-discharged cathodes. And they performed soft XAS at APS beamline 4-ID-C and at DOE’s SLAC National Accelerator Laboratory to observe differences between the surface and the bulk of the nickel in commercial cathode particles.
This new understanding of self-discharge could lead to new designs that could extend the life of batteries. For instance, it might be possible to coat the cathodes with a material that reduces the contact between the reactive surface and the electrolyte. It might also be possible to add ingredients to the electrolyte to stabilize it. – Neil Savage
_____________________________________________________________________________
See: G. Wan1,2, T.P. Pollard3, L. Ma3,4, M.A. Schroeder3, C-C. Chen5, Z. Zhu6, Z. Zhang7, C-J. Sun7, J. Cai7, H.L. Thaman2, A. Vailionis2,8, H. Li2, S. Kelly7, Z. Feng9, J. Franklin10,11, S.P. Harvey12, Y. Zhang13, Y. Du6, Z. Chen7, C.J. Tassone1, H-G. Steinruck1,14,15,16, K. Xu3,17, O. Borodin3, M.F. Toney1,18, “Solvent-mediated oxide hydrogenation in layered cathodes,” Science 385, 6714, 1230-1236 (Sept. 2024)
Author affiliations: 1SLAC National Accelerator Laboratory; 2Stanford University; 3DEVCOM Army Research Laboratory; 4University of North Carolina; 5National Taiwan University; 6Pacific Northwest National Laboratory; 7Argonne National Laboratory; 8Kaunas University of Technology; 9Oregon State University; 10Lawrence Berkeley National Laboratory; 11University College London; 12National Renewable Energy Laboratory; 13University of Houston; 14Universitat Paderborn; 15Institute for a Sustainable Hydrogen Economy; 16RWTH Aachen University; 17SES AI Corporation; 18University of Colorado Boulder.
This work was supported by the Vehicle Technologies Office, funded by the Office of Energy Efficiency and Renewable Energy, US Department of Energy (DOE), including Interagency Agreement IAA 89243322SEE000018 to Army Research Laboratory. The work used DOE Office of Science User Facilities, Advanced Photon Source (no. DE-AC02-06CH11357), Stanford Synchrotron Radiation Lightsource (no. DE-AC02-76SF00515), Environmental Molecular Sciences Laboratory (EMSL) (grid 436923.9, no. 10.46936/sarr.proj.2018.50242/60006384), and Stanford Nano Shared Facilities (National Science Foundation award ECCS-2026822). TOF-SIMS and data analysis performed using EMSL were supported by the DOE, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under award no. 10122. K.X. thanks the Joint Center of Energy Storage Research, an energy hub funded by the US DOE Basic Energy Science, for support. C.-C.C. acknowledges support from the Ministry of Science and Technology of Taiwan (nos. MOST 110-2628-E-002-009 and 111-3116-F-011-006) and the Yushan Young Fellow Program, Ministry of Education of Taiwan (MOE-109-YSFEE-0003-002-P1). J.F. acknowledges support from European Union Horizon 2020 under the Marie Sklodowska-Curie grant. Z.F. acknowledges support from the US National Science Foundation (nos. CBET-1949870 and CBET-2016192) and help from M. Wang and A. Sokolov. G.W. acknowledges assistance from J. E. Mars, C. Cao, and C. J. Takacs.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
