Scientists have identified a plant protein with an unexpected mechanism for producing a class of molecules that have therapeutic potential in various diseases, including cancer. The team of chemists used the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to probe the structure and workings of the protein, which has a unique protein fold. Researchers said that once better understood with further research, it might be possible to harness the chemical processes of the protein to bioengineer new medicines.
Peptides are molecules that contain two or more amino acids. Mainly due to their size, they have several pharmaceutical advantages over small molecules when used as medical drugs, such as increased stability, more specificity and selectivity, greater potency and fewer side effects. Their medical potential has been explored in a wide range of areas, from antimicrobials to anticancer therapies and immunosuppressant treatments for autoimmune diseases.
Cyclic peptides have enhanced pharmaceutical properties compared with linear peptides due to their circular structure, which makes them more stable. This increases their medical potential as they can remain intact longer in patients, making them more promising tools for targeted drug treatments.
Now a team of medicinal chemists at the University of Michigan and the University of Georgia have discovered a plant protein with a novel protein fold and an unusual way of forming cyclic peptides.
The researchers’ field of study is the biosynthesis of larger cyclic peptides found in plants, searching for those with potential as therapeutic drugs. To do this, they study the genetic sequences of plants looking for genes of interest, which enables them to identify novel proteins and the cyclic peptides they produce.
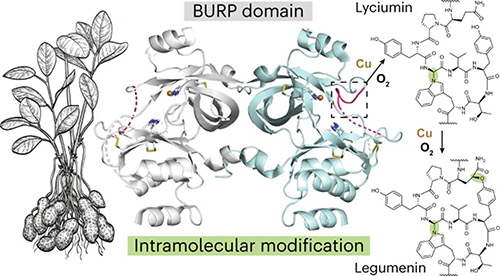
In their latest work, described in Nature Chemical Biology, the team identified a new protein called AhyBURP, which generates cyclic peptides and is found in the roots of the peanut plant.
When the scientists used X-ray crystallography to examine the protein, they discovered that it has a protein fold that has not been seen before and an unexpected method of generating cyclic peptides, which uses copper and oxygen in a unique way. The X-ray crystallography studies of the AhyBURP protein were conducted at beamline 23-ID-B of the APS.
Usually, two proteins work together to take a linear peptide and transform it into a cyclic peptide. But with AhyBURP a single protein performs this process on its own.
The protein also uses copper in the chemical process that creates the cyclic protein. Such copper-dependent proteins normally work with and attach oxygen to the peptide. The AhyBURP protein does not work like this. Uniquely, oxygen is required for the peptide cyclization to work, but the protein does not appear to add oxygen to the cyclic peptide structure.
The researchers do not yet fully understand how the protein uses copper and oxygen in this way or how it transforms peptides with just a single protein and will investigate this in future work. These surprising chemical mechanisms are, however, likely facilitated by the unusual protein fold.
They also plan to screen the cyclic peptides produced by the AhyBURP protein to see how it works against fungi, and more bacteria and cancer cell lines. A cyclic peptide generated by a related protein has been shown in laboratory tests to have anti-cancer properties, providing hope for future medical uses.
Figuring out how the unique chemical mechanisms of the AhyBURP protein function could also pave the way to replicating them, the scientists say. This could enable them to bioengineer their own proteins to synthesize cyclic peptides with specific properties, which could lead to the production of novel therapeutic molecules. – Michael Allen
Read more about this research:
University of Michigan press release
University of Michigan College of Pharmacy press release
See: L.S. Mydy1, J. Hungerford1, D.N. Chigumba1, J.R. Konwerski1, S.C. Jantzi2, D. Wang1, J.L. Smith1, R.D. Kersten1, “An intramolecular macrocyclase in plant ribosomal peptide biosynthesis,” Nat Chem Biol (2024)
Author affiliations: 1University of Michigan; 2University of Georgia.
This study was supported by NIGMS (grant nos. R35GM146934 to R.D.K., F32GM146395 to L.S.M.), NIDDK (grant no. R01DK042303 to J.L.S.), the Hermann Frasch Foundation (R.D.K.) and the PhRMA foundation (predoctoral fellowship, D.N.C.). We thank the University of Michigan Center for Structural Biology and E. Scott for crystallography resources and for fast protein liquid chromatography (FPLC) use. We appreciate T. Cernak for access to synthetic resources, B. Palfey for spectrophotometer and fluorometer access, J. Bridwell-Rabb for glove box access and M. Knapp for training L.S.M. We are grateful to T. Cernak, B. Palfey and J. Bridwell-Rabb for helpful discussions as well. We also thank G. Lomonosoff (John Innes Centre, UK) for sharing the pEAQ-HT vector. GM/CA at the Advanced Photon Source has been funded by the National Cancer Institute (grant no. ACB-12002) and the National Institute of General Medical Sciences (grant nos. AGM-12006, P30GM138396). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. The Eiger 16M detector at GM/CA-XSD was funded by a National Institutes of Health grant no. S10 OD012289. L.S.M. thanks Q. Xu for aid in determining anomalous difference density.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
