Quantum materials are increasingly being explored for their exotic properties. A fundamental feature of these compounds is that scientists cannot employ conventional rules to describe their electronic behavior. Instead, complex quantum interactions such as electron correlations must be accounted for.
One fascinating phenomenon called an electronic flat band can occur in quantum materials with just the right geometric structure. These bands consist of many electrons with nearly the same kinetic energy, which enhances electron-electron interactions that produce unusual and technologically important behavior. Up until now flat bands were only confirmed in graphene and other 2D structures. The extension of electronic flat bands into three dimensions would significantly aid further investigation of this quantum state, with many potential applications including high-temperature superconductivity and fractionalized topological states.
In this research, scientists endeavored to create flat bands in three-dimensional materials. They did this by synthesizing calcium-nickel (CaNi2) crystals featuring a star-shaped pattern called a pyrochlore lattice. This lattice is related to the 2D hexagonal shape that has previously been shown to produce two-dimensional flat bands. The researchers detected flat bands in a three-dimensional pyrochlore lattice using sophisticated
techniques, including angle-resolved photoemission spectroscopy (ARPES) performed at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
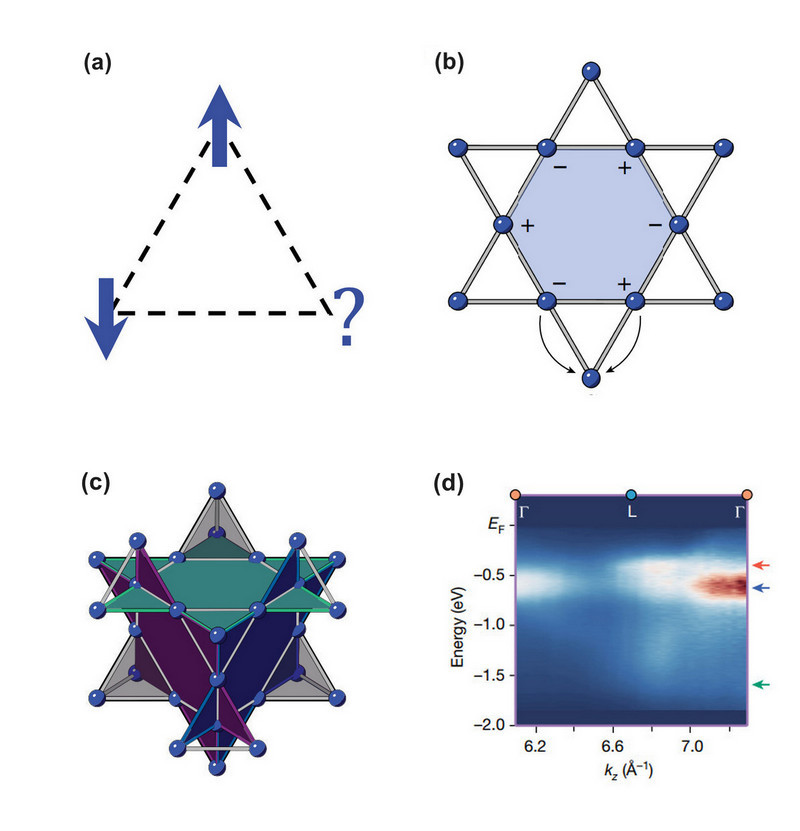
Unusual quantum behavior can be driven by geometric frustration, which only occurs for particular arrangements of the atoms composing a lattice. Figure 1a involves a triangular arrangement of three atoms with arrows indicating the spin of each atom's outermost electron. If an interaction induces the spins to point in opposite directions, as shown on the triangle’s top and left side, then the spin of the third electron is indeterminate or “frustrated” because the overall energy is the same whether the third electron spins up or down. This frustration can lead to quantum entanglements between the electrons.
The flat bands studied here arise from this geometric induced electron correlation, whereby quantum interference prevents electrons from escaping a hexagon of atoms referred to as a kagome pattern (Fig. 1b). Unlike trivially localized states like the one in Fig. 1a, these flat-band-localized states strongly overlap one another and promote quantum entanglement and correlations. While flat bands have been shown to occur in a variety of 2D materials including twisted graphene and crystalline 2D kagome lattices, producing electronic flat bands in thick crystals is significantly more challenging due to the difficulty of enforcing correlation in three dimensions. The researchers overcame these difficulties by selecting and then synthesizing pure 3D crystals of CaNi2.
The CaNi2 compound is classified as a Laves phase mineral, and it features interpenetrating kagome layers that form a pyrochlore lattice (Figure 1c). While the ARPES imaging technique can readily detect flat bands in 2D lattices, it is challenging to use on 3D crystals. This challenge was met by mirco-focusing the ARPES beams to an area around one-hundred-thousandth of a square millimeter (about 20x30 square micrometers) and aiming the beam at locally flat surfaces exposed by cleaving the crystal.
Soft (lower-energy) X-rays were utilized for the ARPES measurements performed at beamline 29-ID of the APS. Complimentary ARPES experiments were performed at DOE’s Lawrence Berkeley National Laboratory and DOE’s Brookhaven National Laboratory using high-energy ultraviolet radiation. The collective ARPES data revealed that the nickel electrons in the CaNi2 compound hosted bands that are very flat (Fig. 1d). This behavior is unusual because such bands would normally bend or “disperse” in most materials if not for the electronic correlation.
The CaNi2 samples were also probed using other experimental techniques to reveal their magnetic, thermodynamic, and other properties. Additionally, researchers synthesized another promising pyrochlore by replacing nickel with the element rhodium (Rh) and adding a small proportion of ruthenium (Ru). Thermodynamic and ARPES measurements revealed the calcium-rhodium-ruthenium pyrochlore contains the same flat bands and becomes a superconductor.
The researchers point out that the kagome structure can be realized in many types of pyrochlores besides the two examined here, as well as in non-pyrochlore compounds. This flexibility will allow scientists to synthesize custom crystals to investigate a wide range of quantum phenomena in 3D materials. – Philip Koth
____________________________________________________________________________________
See: J.P. Wakefield1, M. Kang1,2, P.M. Neves1, D. Oh1, S. Fang1, R. McTigue1, S.Y.F. Zhao1, T.N. Lamichhane1, A. Chen1, S. Lee2,3, S. Park2,3, J.-H. Park2,3, C. Jozwiak4, A. Bostwick4, E. Rotenberg4, A. Rajapitamahuni5, E. Vescovo5, J.L. McChesney6, D. Graf7, J. Palmstrom7, T. Suzuki8, M. Li1, R. Comin1, J.G. Checkelsky1, “Three-dimensional flat bands in pyrochlore metal CaNi2,” Nature 623 301-306 (November 2023)
Author affiliations: 1Massachusetts Institute of Technology; 2Max Planck POSTECH/Korea Research Initiative; 3Pohang University of Science and Technology; 4Lawrence Berkeley National Laboratory; 5Brookhaven National Laboratory; 6Argonne National Laboratory; 7National High Magnetic Field Laboratory; 8Toho University
This work was funded, in part, by the Gordon and Betty Moore Foundation EPiQS Initiative (grant no. GBMF9070 to J.G.C.) (instrumentation development, DFT calculations), the Air Force Office of Scientific Research (AFOSR) (award FA9550-22-1-0432) (material synthesis, ARPES) and the NSF (DMR-2104964) (material analysis) and the Center for Advancement of Topological Semimetals, an Energy Frontier Research Center funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), through the Ames Laboratory (contract no. DE-AC02-07CH11358) (pulsed-field experiments). P.M.N. acknowledges support from the STC Center for Integrated Quantum Materials (NSF grant DMR-1231319). M.K., S.L., S.P. and J.-H.P. acknowledge support from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (nos 2022M3H4A1A04074153 and 2020M3H4A2084417). M.L. acknowledges support from the DOE, BES (DE-SC0020148). T.S. acknowledges support from JSPS KAKENHI (grant no. 21K03454). This research used resources of the Advanced Light Source, which is a DOE, Office of Science, user facility (contract no. DE-AC02-05CH11231). This research used resources from the Advanced Photon Source, a DOE, Office of Science, user facility operated for the DOE, Office of Science, by the Argonne National Laboratory (contract no. DE-AC02-06CH11357). A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation Cooperative Agreement no. DMR-1644779, the State of Florida and the DOE. We thank the MIT SuperCloud54 and the Lincoln Laboratory Supercomputing Center for providing high-performance computing resources that have contributed to the results reported in this study.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
