Transformation of carbon dioxide into useful industrial chemicals once required rare, expensive noble metals such as gold, silver, and palladium to act as catalysts. Now a newer generation of carbon-based electrocatalysts are under development. These more affordable materials have large surface areas and use carbon structures such as graphene doped with sulfur, nitrogen, or oxygen to tune their electronic structures to produce specific chemicals from the reaction.
Carbon dioxide (CO2) and carbon monoxide (CO) are the most ubiquitous products of burning fossil fuels, and many large power plants, cement manufacturers, breweries and other industries produce copious streams of them. Having a cheap, efficient way to turn those streams of CO2 and CO into useful chemicals would be enormously valuable. The trick is to break the carbon-oxygen bonds in a chemical process called reduction reaction, which leaves the carbon with one or more unpaired electrons that are then available to react with other atoms.
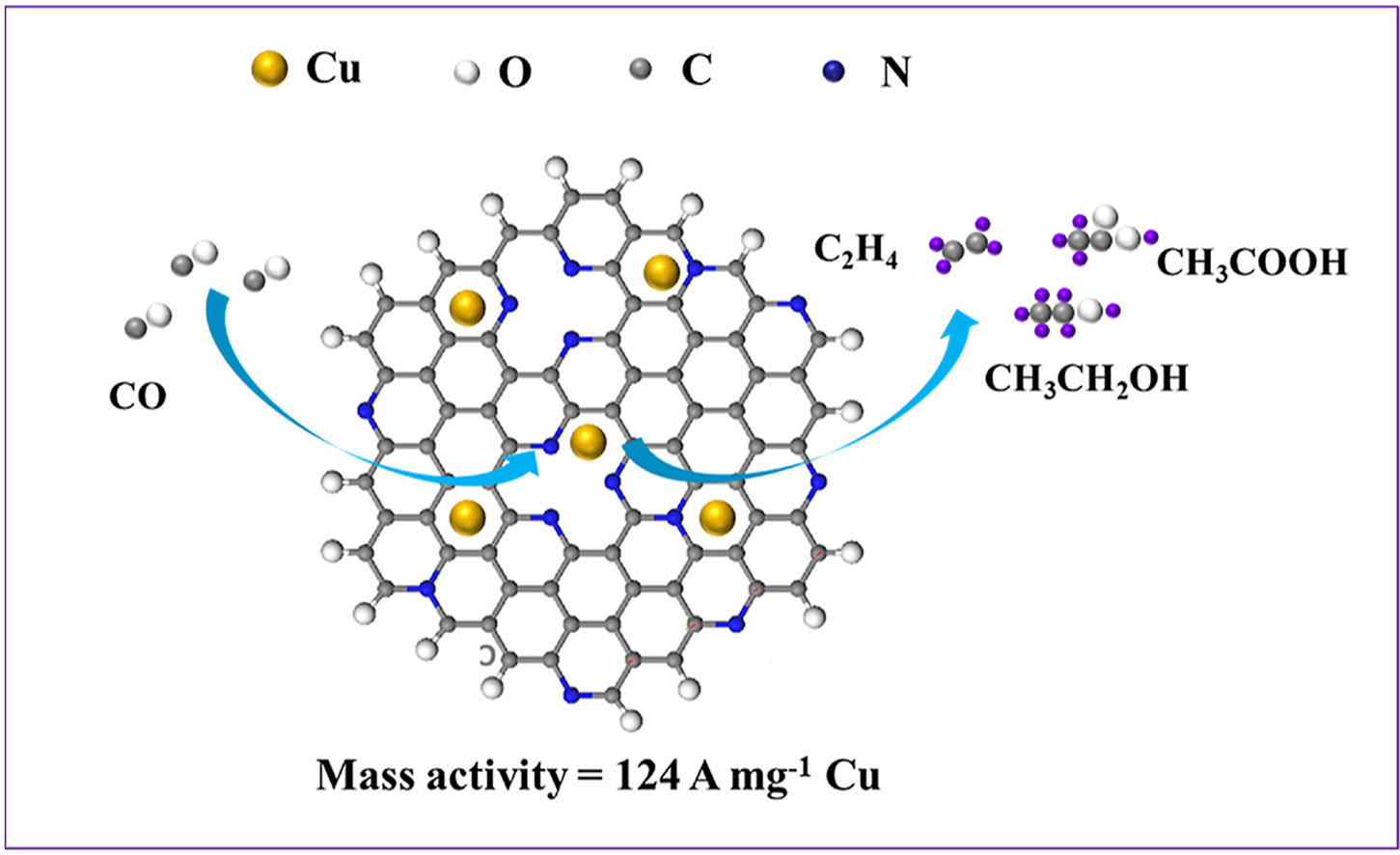
Nitrogen-doped graphene quantum dots can act as catalysts for reduction reaction. They are essentially flat sheets of carbon in a hexagonal, honeycomb lattice, studded with the occasional nitrogen atom sporting a functional group such as oxygen and hydrogen (OH-), or nitrogen dihydrogen (NH2-). When the nitrogen-doped graphene quantum dots are used as a catalyst, the functional groups can donate electrons as needed to facilitate the break-up of CO2 or CO. They can also facilitate the creation of specific chemicals—in general, nitrogen-doped graphene quantum dots tend to produce methane (CH4) from both CO and CO2.
But if you add trace bits of copper to the situation, the balance of chemicals produces shifts. In particular, if most of the feedstock is CO, adding trace amounts of copper shifts the chemical products away from CH4 and toward compounds with two or more carbons. A team of researchers from the University of Cincinnati, Oak Ridge National Laboratory, and Argonne National Laboratory used the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to investigate just how the amount of trace copper correlates to the balance of products produced in both CO and CO2 reduction reaction.
The researchers prepared nitrogen-doped graphene quantum dots containing trace amounts of copper ranging from a low of 0.056µg/cm2 to a high of 14.1µg/cm2. They placed the quantum dots on electrodes and then ran either a CO reduction reaction or a CO2 reduction reaction, and measured the chemical products produced. The team found that trace quantities of copper on the quantum dots in a CO reduction significantly shifted the products towards compounds with two or more carbons.
The same effect occurred when trace copper was added to the graphene quantum dots in the CO2 reduction reaction, but was not as pronounced, with the dominant product being C2H4. The difference in products suggests that the trace copper favored different reaction pathways in CO2 reduction than in CO reduction.
For clues as to why the copper favored C2 compounds, the researchers took X-ray absorption near-edge structure (XANES) spectroscopy measurements of the electrodes at APS beam line 20-ID-B. The XANES spectroscopy suggested that in the nitrogen-doped graphene quantum dots containing trace copper, the copper atoms were in a chemical oxidation state somewhere between +1 and +2. This could encourage CO reduction reaction to create C2 compounds in several ways. The two most likely are that either the copper encourages carbon-carbon bonds directly, or that the copper works together with a neighboring site on the catalyst—most likely a nitrogen—to drive carbon-carbon coupling.
This work shows that the presence and contribution of trace levels of copper must be considered when designing metal-free catalysts for CO2 production. The researchers are now considering how to design and synthesize highly efficient, selective, and durable CO2 and CO reduction reaction catalysts with low levels of copper. – Kim Krieger
___________________________________________________________________________
See: X. Lyu1,2, T. Zhang1, Z. Li1, C.J. Jafta2, A. Serov2, I.-H. Hwang3, C. Sun3, D.A. Cullen2, J. Li2, J. Wu1, “Trace Level of Atomic Copper in N-Doped Graphene Quantum Dots Switching the Selectivity from C1 to C2 Products in CO Electroreduction,” Mat Today Chemistry 29 101398 (2023)
Author affiliations: 1University of Cincinnati; 2Oak Ridge National Laboratory; 3Argonne National Laboratory.
This work is partially supported by the Office of Fossil Energy and Carbon Management of the U.S. Department of Energy under Award Number DE-FE0031919. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory and was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
