 Stored fat has a good and bad side. It supplies energy when food is scarce, but it can lead to adverse health conditions when too much is stored. Using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, a team of researchers has uncovered an interaction between two molecules that may repress fat-burning under high-fat diet.
Stored fat has a good and bad side. It supplies energy when food is scarce, but it can lead to adverse health conditions when too much is stored. Using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, a team of researchers has uncovered an interaction between two molecules that may repress fat-burning under high-fat diet.
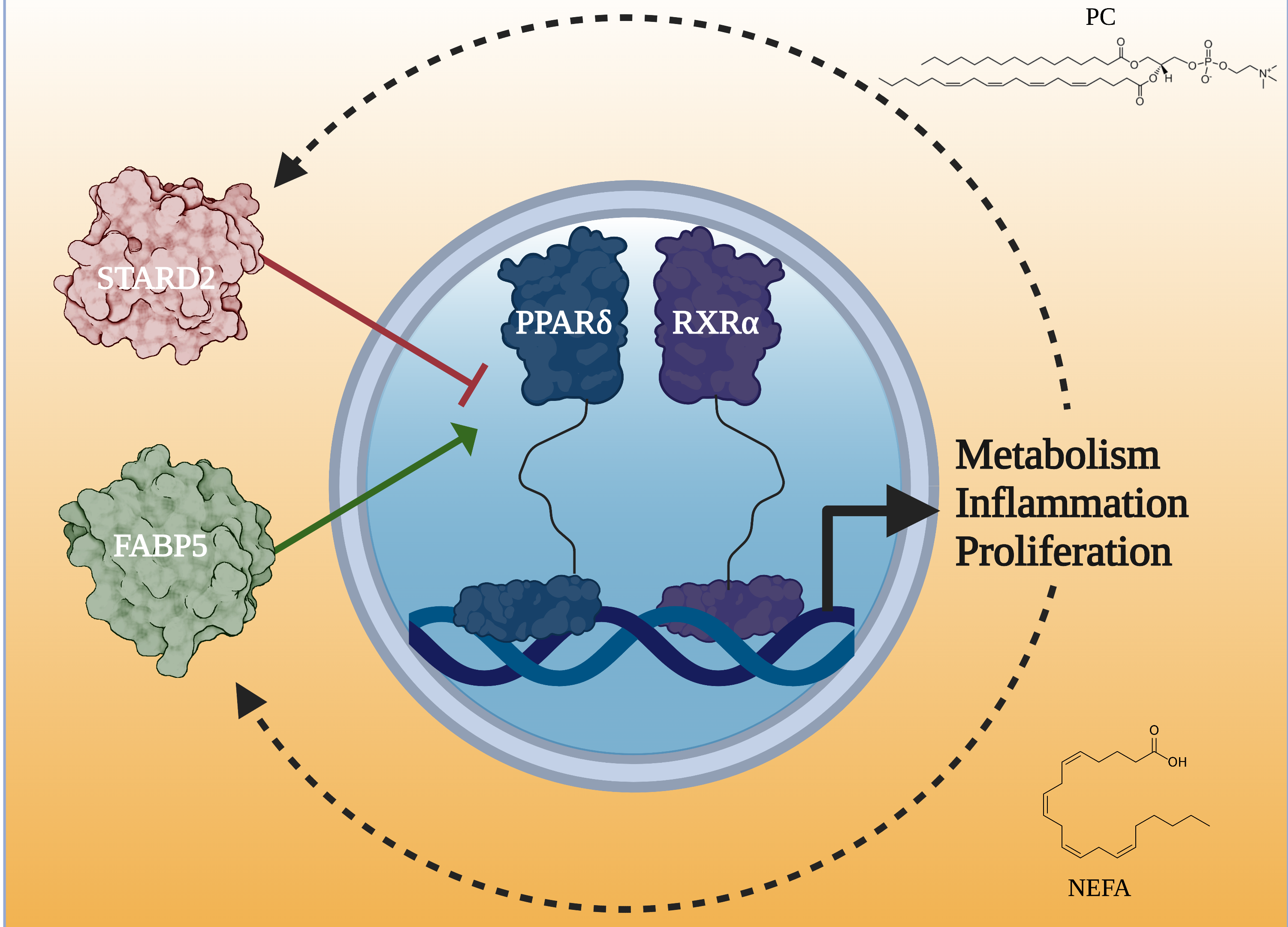
One of those molecules, phosphatidylcholine transfer protein (PCTP), suppresses the activity of the other, PPARdelta, a gene regulator critical in burning fat and energy metabolism, under conditions of overnutrition. Their research shows that phosphatidylcholines can act as signaling molecules and that PPARdelta can be targeted indirectly, by inhibiting PCTP. The research, published in Nature Communications, may open the door for small molecule drugs that can help fight obesity and other metabolic conditions without the side effects of directly activating PPARdelta, including cancer.
Unlike modern humans, who have constant access to food, our ancestors endured long periods of fasting between eating. To yield off starvation, they evolved the ability to store fat, which was burned for energy when food was scarce. However, when food is abundant, stored fat can lead to adverse health conditions, such as obesity, fatty liver disease, and diabetes.
Our bodies have many ways of burning fat, such as the transportation of fatty acids, derived from food, into mitochondria for fatty acid oxidation. This is one of many tasks regulated by lipid transport proteins such as PCTP.
However, scientists found that in a state of overnutrition (aka overeating), PCTP can play a pathogenic, rather than productive, metabolic role. In this study, they show that PCTPs can repress the activity of a nuclear receptor called PPARdelta, a gene regulator critical in burning fat, maintaining energy balance, and regulating fatty acid uptake. Repress the gene that burns fat, and fat is stored instead, they hypothesize.
The researchers did not intend to study PCTPs in particular—they were interested in investigating how nuclear receptors get activated. To that end, they conducted a large screen crossing lipid-sensing nuclear receptors and soluble lipid transporters. One pair stood out: lipid transporter PCTP and nuclear receptor PPARdelta. Their interaction was strong, selective, and new: PPARdelta was known to interact with lipid transport proteins but not phosphatidylcholine transport proteins.
The scientists first created a whole-body PCTP knockout male mouse (meaning one in which genes have been activated or deactivated), assessing its fat metabolism under different dietary conditions, including normal and high-fat diet. Under normal diet, knockout had very little effect, but under high-fat diet, knockout mice showed a marked decrease in body weight and fat mass, as well as beneficial alterations in glucose and insulin sensitivity.
Such stark differences in phenotype led the scientists to make a liver-specific PCTP knockout. This would allow them to focus on mechanism while defraying the possibility that the knockout effect could be acting through other tissue, such as muscle or heart. The results were the same: a stark difference between the knockout and wild-type mice under high-fat conditions.
Finally, the researchers focused on the specific protein interactions driving the different phenotypes. Gene expression analysis suggested that the differences derived from repression of PPARdelta gene regulation activity. An in-cell protein complementation screen uncovered a direct interaction between PCTP and PPARdelta that was not observed for other PPARs.
A crucial step in the process was directly linking phospholipids to the suppression of PPARdelta. The scientists solved the structure of PCTP bound to phosphatidylcholine, collecting crystallographic data at the Southeast Regional Collaborative Access Team (SER-CAT) beamline at 22-ID at the APS.
Solving the crystal structure enabled them to make mutations in PTCP’s binding pocket. The mutations reduced lipid binding, which reduced PTCP’s ability to repress PPARdelta. From that they were able to infer that lipid binding was critical to support the mechanism driving PPARdelta repression. It’s not a smoking gun—they would require very sophisticated mass spectrometry or kinetics to look at the transfer of phospholipid from one protein to another—but the researchers were able to support their hypothesis that the interaction between PCTP and PPARdelta is repressive, and when PCTP is unable to bind phosphatidylcholines, it is unable to interact with and repress PPARdelta.
The research points to a novel regulator of nuclear receptor activity, and possibly a potential way to target PPARdelta indirectly. Other labs have targeted PPARdelta directly for its fat-burning and glucose metabolic abilities, creating a “marathon mouse” that ran twice as far as other mice and lost weight on a high-fat diet. However, PPARdelta also plays a major role in proliferation, and activating this receptor with small molecules can lead to various forms of cancer. Targeting PPARdelta indirectly could be of great benefit to people fighting obesity and insulin insensitivity. The researchers will next attempt to collaborate with other investigators on improving small molecule inhibitors of PCTP. – Judy Myers
____________________________________________________________________________________
See: S. A. Druzak1, M. Tardelli2, S. G. Mays1, M. E. Bejjani1, X. Mo1, K. M. Manner-Smith1, T. Bowen1, M. L. Cato1, M. C. Tillman1, A. Sugiyama2,3, Y. Xie2,3, H. Fu1, D. E. Cohen2,3, E. A. Ortlund1, “Ligand dependent interaction between PC-TP and PPARdelta mitigates diet-induced hepatic steatosis in male mice,” Nat Commun. 14, 2748 (2023)
Author affiliations: 1Emory University School of Medicine; 2Weill Cornell Medical College; 3Brigham and Women’s Hospital, Harvard Medical School.
The authors would like to thank Dr. Hongliang Li from the Institute of Model Animal (IMA), Wuhan University for the generous donation of the Pctp. Crystallographic data were collected at Southeast Regional Collaborative Access Team 22-ID Beamline at the Advanced Photon Source, Argonne National Laboratory. SER-CAT is supported by its member institutions and equipment grants (S10_RR25528, S10_RR028976 and S10_OD027000) from the National Institutes of Health. Use of the Advanced Photon Source was supported by the Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract W-31–109-Eng-38. Finally, the authors would like to thank Pooja Srinivas, Jen Colucci, and Emma D’Agostino for assistance with editing of the manuscript. A F31 training fellowship from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK), F31 DK 126435 and training grant T32 GM 008602 supported S.A.D during the duration of this work. This work was supported by the NIH (R01 DK 048873, DK 056626 and DK 103046 to D.E.C. and E.A.O.). M.L.C. was supported by T32 GM 008367-29 This study was supported in part by the Emory Integrated Metabolomics and Lipidomics Core, which is subsidized by the Emory University School of Medicine, and is one of the Emory Integrated Core Facilities. Additional support was provided by the Georgia Clinical and Translational Science Alliance of the NIH, Award UL1 TR 002378. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
