 When our phone blinks its 1% power warning, we reach for a charger. Ideally, the charger would replenish from a green source, such as solar or wind, but these produce energy only intermittently. One alternative for this energy source is a hydrogen fuel cell. But how to generate the hydrogen stored within the cell in a green manner?
When our phone blinks its 1% power warning, we reach for a charger. Ideally, the charger would replenish from a green source, such as solar or wind, but these produce energy only intermittently. One alternative for this energy source is a hydrogen fuel cell. But how to generate the hydrogen stored within the cell in a green manner?
Electrolysis, which splits water molecules to produce hydrogen, could use renewable resources to provide it. However, electrolysis requires a catalyst that increases the rate of the key chemical reaction at its anode, called the oxygen evolution reaction. Perovskite-type minerals are environmentally friendly and fairly efficient catalysts for this reaction, but to improve the rate for widespread use, scientists need to understand which characteristics most influence the catalyst's efficiency. Currently, material scientists are investigating and comparing two characteristics: the average amounts of the constituent elements in a specified unit of its volume (its bulk composition) and the specific quantities of ions present on the surface involved in the key chemical reaction.
Recently, researchers from multiple Pacific Northwest academic institutions and a number of U.S. national laboratories have demonstrated that although tuning the bulk composition of the catalyst improves an electrolyser's efficiency, the transformation of the catalyst surface under electrolysis conditions is what drives the increase in oxygen evolution reaction activity.
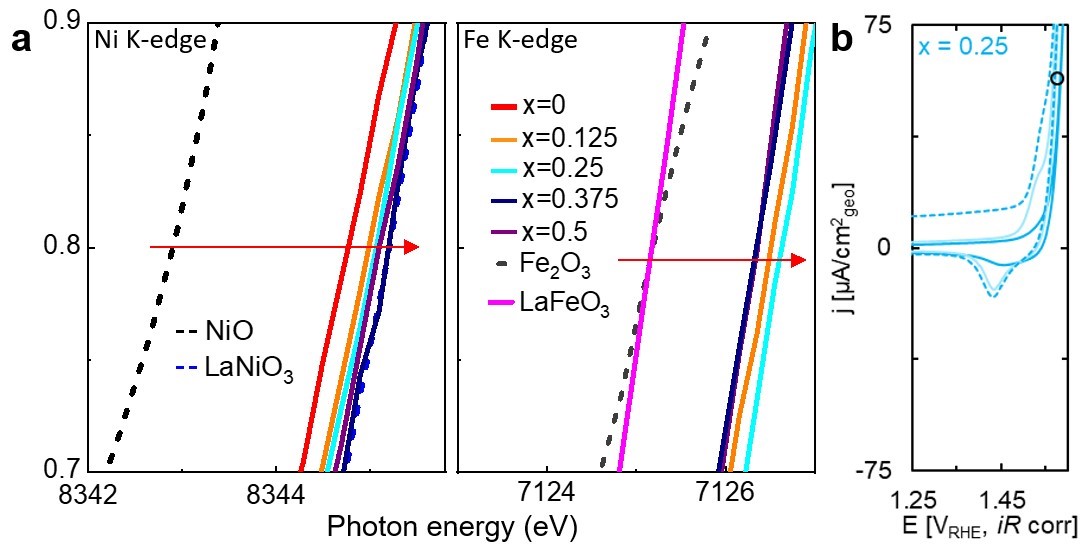
The researchers modified lanthanum nickel oxide to incorporate both iron and strontium into its structure. The team leveraged previously published studies showing these changes improved oxygen evolution reaction activity during electrolysis. The team produced five different crystalline films, varying the amount of iron and nickel in each. They used multiple X-ray investigative methods to characterize the thin film samples, including nickel and iron K-edge X-ray absorption near-edge spectra using beamline 9-BM at the Advanced Photon Source (APS). These measurements showed that the nickel oxidation state is slightly less than 3+ and the iron oxidation state is nominally greater than 3+ for all compositions, as illustrated in Figure 1A.
To test the influence of the samples' surface properties, the team subjected each of the five samples to a sequence of voltage profiles that drive the oxygen evolution reaction and probe different reduction-oxidation processes of the surface ions. This sequence of voltage profiles is an example of electrochemical cycling.
The team found that oxygen evolution reaction activity increased with the amount of time a film spent exposed to cyclic voltammetry. Films with larger amounts of iron produced higher current densities during the cyclic voltammetry where the voltage window included the reduction and oxidation of iron. These iron-rich films also showed that the response of nickel evolved with the amount of time exposed to cyclic voltammetry. This is especially significant as other research reports similar nickel behavior occurs in the presence of a nonuniform distribution of iron. To clarify the role of the samples' surfaces in these results, the team also examined the surfaces with other methods.
First, the team characterized the surface of a sample after cyclic voltammetry to see how the amounts and oxidation states of strontium, iron and nickel had changed. They chose the sample with the second-highest amount of iron. Using multiple X-ray and microscopy investigative techniques, the team determined that the surface of the film became more rough during cyclic voltammetry, resulting in a larger number of exposed active sites. Researchers concluded the increase in active sites is partially responsible for the observed increase in oxygen evolution reaction activity. The team also found that the composition of the film surface altered with the amount of time exposed to voltage changes, including a reduction in strontium content. Additionally, the team found that the nickel on the samples' surfaces was segregated into regions rather than uniformly distributed. This correlates with the previously observed evolution of nickel's response to cyclic voltammetry in samples with higher iron content.
The team also used the charge measured from all five samples during the voltage changes (shown in Figure 1B) to estimate the number of nickel atoms per square nanometer in the films. The nickel estimates were smaller than expected during the first round of cyclic voltammetry but doubled during the second round. However, since the oxygen evolution reaction activity only increased in samples with larger amounts of iron, the team concluded that the covalency of the iron-oxygen sites is critical to enhancing oxygen evolution reaction activity, not the average amount of nickel in the sample (the bulk composition).
The researchers also observed that the local environment of the active iron sites could change notably during measurement: when bulk iron is present, it may redistribute and re-deposit during cyclic voltammetry. Since these iron re-distribution changes occur with increased oxygen evolution reaction activity, this is another example of how the surface properties, and not the bulk composition, influences oxygen evolution reaction activity. The team concluded that although bulk composition can provide a template for understanding trends in oxygen evolution reaction activity, it is the dynamic nature of the surface composition of a perovskite-type mineral with alkaline-earth A-sites, when responding to electrochemical cycling, that drives the mineral's intrinsic oxygen evolution reaction activity.
The team's measurements show how the incorporation of strontium and iron into lanthanum nickel oxide improves the perovskite-type mineral's ability to generate hydrogen more efficiently. Additionally, by examining the surface behavior as a function of exposure to voltage changes, the team showed how the behavior of the ions at the electrolyzed surface drives oxygen evolution reaction activity---and perhaps someday, our charging stations. – Mary Agner
______________________________________________________________________________________
See: P. Adiga1, L. Wang2, C. Wong1, B. E. Matthews2, M. E. Bowden2,S. R. Spurgeon2,3, G. E. Sterbinsky4, M. Blum5, M-J. Choi2, J. Tao2, T. Kaspar2, S. A. Chambers2, K. A. Stoerzinger1,2, Y. Du2, “Correlation between oxygen evolution reaction activity and surface compositional evolution in epitaxial La0.5Sr0.5Ni1−xFexO3−δ thin films,” Nanoscale, 2023,15, 1119-1127 (2023)
Author affiliations: 1Oregon State University; 2Pacific Northwest National Laboratory; 3University of Washington, 4Argonne National Laboratory, 5Lawrence Berkeley National Laboratory.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
