 Producing alcohol from captured carbon emissions could help fight climate change and aid the burgeoning renewable carbon economy. Using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility, a group of scientists have shown that adding barium oxide to catalysts can significantly increase the production of alcohol over unwanted byproducts. Their results were published in the journal Nature Catalysis.
Producing alcohol from captured carbon emissions could help fight climate change and aid the burgeoning renewable carbon economy. Using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility, a group of scientists have shown that adding barium oxide to catalysts can significantly increase the production of alcohol over unwanted byproducts. Their results were published in the journal Nature Catalysis.
Carbon capture, utilisation and storage is seen by many as vital for meeting future energy and climate goals. Carbon dioxide (CO2) is captured from the air or from industrial processes and then sent to underground storage sites, such as depleted offshore oil fields, or converted into fuels and chemicals.
As well as helping tackle climate change, CO2 utilisation could create a profitable carbon economy. There are several techniques for converting CO2 into useful chemicals and fuels, including electrochemical (or electrocatalytic) reduction. The most desirable alcohol product from this process is ethanol.
Ethanol is commonly added to gasoline for cars, replacing a proportion of the petrochemical fuel, and it can also be converted to sustainable aviation fuel, via an industrial process known as ethanol-to-jet. Ethanol can be turned into a wide range of other useful chemicals, including ethylene, which is used to make various polymers such as the polyethylene plastic.
The electrocatalytic reaction takes place in an electrolysis cell in which the cathode and the catholyte (the cathode liquid) are separated by a membrane from the anode and the anolyte. During the catalytic process the electric current drives the reduction of CO2 at the catalyst (the cathode) through electron transfer and proton transfer. As well as alcohols like ethanol and methanol, the chemical compounds resulting from this process include unwanted hydrocarbons.
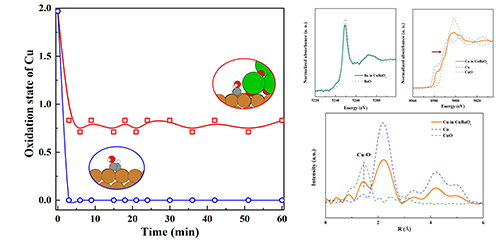
Controlling the reaction to produce alcohols over hydrocarbons remains a key challenge in the electrochemical reduction of CO2. Oxidized copper catalysts have been shown to increase the conversion of CO2 into alcohols, particularly ethanol. But oxidized copper rapidly reduces and loses oxygen during the electrochemical process. This means it become less reactive and loses its ability to selectively produce alcohols.
In the Nature Catalysis paper, an international team of scientists hypothesized that adding metal oxides, which maintain their oxidation states for long periods during electrochemical reactions, to a copper catalyst would create a more stable copper oxygen interface. The hope was that a metal oxide decorated copper catalyst would have the same selectivity for alcohols as a copper oxide catalyst but remain reactive for much longer.
The team created catalysts of copper and three different metal oxides: barium oxide, strontium oxide and calcium oxide. They then performed electrochemical reductions with these catalysts while using several different methods to analyse the chemicals produced, the structure of the catalyst and its stability, and the chemical reactions underlying the process. These included various electron microscopy and spectroscopy techniques, including X-ray absorption spectroscopy, which was carried out at beamline 9-BM of the APS, an Office of Science user facility at Argonne National Laboratory.
The researchers found that adding metal oxides to copper catalysts did promote the conversion of CO2 to alcohol. In particular, the barium oxide and copper catalyst demonstrated a 2.5-fold higher selectivity for alcohol than pure copper.
The barium oxide and copper catalysts were also found to be stable. X-ray absorption spectroscopy showed that during the electrochemical reduction of CO2 these catalyst precursors partially reduce within three minutes but then maintained a steady oxidation state, while copper oxide catalysts rapidly reduced to copper. The X-ray absorption spectroscopy also indicated an abundance of barium oxide and copper interfaces that created arrangements of copper and oxygen molecules beneficial to the reduction of CO2.
Further analysis explained the enhanced selectivity for alcohols. It showed that the copper and oxygen interfaces affected the strength of different bonds in various chemical reactions pushing the products towards alcohols, such as ethanol, rather than hydrocarbons. In particular, the interfaces stabilise and favour the formation of hydroxy groups, which are the key functional group of alcohols, over groups that go on to form hydrocarbons.
These findings around the promotion of alcohol selectivity could pave the way to improvements in electrocatalyst design to enhance alcohol production from CO2. – Michael Allen
______________________________________________________________________________________
See: A. Xu1,2,3,, S-F. Hung1,4, A. Cao3, Z. Wang3, N. Karmodak3, J. E. Huang1, Y. Yan1, A. S. Rasouli1, A. Ozden1, F-Y. Wu4, Z-Y. Lin4, H-J. Tsai4, T-J. Lee4, F. Li1, M. Luo1, Y. Wang1, X. Wang1, J. Abed1, Z. Wang1, D-H. Nam1, Y. C. Li1,5, A. H. Ip1, D. Sinton1, C. Dong4, E. Sargent1, “Copper/alkaline earth metal oxide interfaces for electrochemical CO2 to alcohol conversion by selective hydrogenation,” Nat Catal 5, 1081–1088 (2022)
Author affiliations: 1University of Toronto; 2University of Science and Technology Beijing; 3Technial University of Denmark, 4National Yang Ming Chiao Tung University, 5University of Buffalo
This work was financially supported by Suncor Energy, the Natural Sciences and Engineering Research Council (NSERC) of Canada and the CIFAR Bio-Inspired Solar Energy program. S.-F.H. acknowledges support from the Ministry of Science and Technology, Taiwan (contract nos MOST 110-2113-M-009-007-MY2, MOST 110-2628-M-A49-002 and MOST 111-2628-M-A49-007) and from the Yushan Young Scholar Program, Ministry of Education, Taiwan. This research used the synchrotron resources of the Advanced Photon Source (APS), an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, and was supported by the US DOE under contract no. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
