 Rare-earth (RE) sesquioxides, oxides formed by a subset of lanthanide elements, have attracted increasing attention for their unique fundamental properties. These properties – including a high quantum yield, biocompatibility, high thermal and chemical stability, and resistance to phase transformation upon irradiation in some cases – could be harnessed for a variety of different applications. These include biomedical applications such as in drug delivery and cell tracking and labeling; electronic applications such as electrochemical and electroluminescent sensors; and radiation-resistant materials for applications in reactors.
Rare-earth (RE) sesquioxides, oxides formed by a subset of lanthanide elements, have attracted increasing attention for their unique fundamental properties. These properties – including a high quantum yield, biocompatibility, high thermal and chemical stability, and resistance to phase transformation upon irradiation in some cases – could be harnessed for a variety of different applications. These include biomedical applications such as in drug delivery and cell tracking and labeling; electronic applications such as electrochemical and electroluminescent sensors; and radiation-resistant materials for applications in reactors.
Mixing five or more REs in equiatomic amounts has been shown to result in single-phase RE2O3 with promising properties that could surpass the performance of single-RE oxides. However, although single-RE oxides have been shown to undergo predictable polymorphic transitions with changing temperature, such polymorphic transitions and thermal expansion of multi-RE oxides have not been well documented. To fill this gap, researchers at the University of Tennessee, Oak Ridge National Laboratory, and Argonne National Laboratory used beamline 6-ID-D at the Advanced Photon Source (APS) to investigate how the structures of two different RE sesquioxides that incorporated unique combinations of five RE cations in equimolar amounts changed as they cooled from a melted state.
The researchers designed two different multi-RE sesquioxides for this study based on their average ionic radius (AIR), which was determined in previous studies to influence RE2O3 behavior. Composition 1 contains a mixture of small and large REs, for an AIR close to the ionic radius of Gd3+; composition 2 contained a mixture of medium and large, for an AIR close to that of Pm3+, although Pm3+ was not included in either formulation.
They prepared powder samples of these two compositions using a wet chemistry synthesis method, then subjected them to room-temperature powder X-ray diffraction (XRD) to determine their structures. Composition 1 and 2 closely matched the structures of C-type Gd2O3 and Pm2O3 respectively, as expected based on their AIRs.
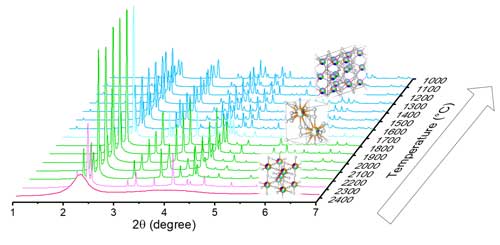
The structural evolution of each composition under high temperatures was studied using synchrotron X-ray diffraction (XRD) at the APS. To avoid the need to use a container to house these samples at extreme temperatures, the researchers prepared bead samples for aerodynamic levitation by melting the powders to form 2- to 3-mm beads. Room temperature XRD showed a single monoclinic B-type structure for the as-prepared beads of both formulations, indicating that a phase transition had taken place from the C-type phase seen in the powders.
However, when the researchers heated these beads with a laser to melting temperatures above 2400 Celsius, they saw a departure in their phase evolutions. As composition 1 cooled, it transitioned from a liquid to a single H-type phase at ~2350 °C to a single B-type phase at ~2150 °C. This B-type phase was stable upon cooling to 1000 °C. Both the melting and B- to H-type were close to the smaller end of the temperature range previously reported for Gd2O3, as expected based on composition 1’s AIR close to this single-RE sesquioxide.
In contrast, as composition 2 cooled, it transitioned from a liquid to a mixture of X- and H-type between 2350 and 2300 °C, to single H-type between 2250 and 1800 °C, to a mixture of H- and B-type at 1750 °C, and a single B-type phase between 1700 and 1000 °C. This transformation differs significantly from the polymorphism reported for Pm2O3, even though the AIR of composition 2 is similar.
When the researchers determined the coefficient of thermal expansion (CTE) for both compositions, a measure of how a material expands with temperature, they found that composition 1’s CTE closely matched that of Gd2O3, much as its polymorphic behavior did. Composition 2’s CTE also matched the CTE of a relatively simple oxide of the same structure, YbFeO3.
The authors suggest that sesquioxides with RE combinations other than the ones reported here could have surprisingly different polymorphic and thermal expansion behaviors, a topic for future studies. – Christy Brownlee
See: M. Pianassola1,, K. Anderson1, C. Agca2, C. J. Benmore3, J. W. McNurray2, J. C. Neuefeind2, C. Melcher1, M. Zhuravleva1,“In Situ High-Temperature Structural Analysis of High-Entropy Rare-Earth Sesquioxides,” Chem. Mater. 2023, 35, 3, 1116–1124 (January 2023)
Author affiliations: 1University of Tennessee Knoxville; 2Oak Ridge National Laboratory; 3Argonne National Laboratory.
This work was supported by the National Science Foundation (DMR 1846935). This material is partially based upon work supported by the U.S. Department of Homeland Security under grant award number 20CWDARI00037-01-00. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Department of Homeland Security. Powder XRD was performed at the Institute for Advanced Materials & Manufacturing (IAMM) Diffraction Facility, located at the University of Tennessee, Knoxville. A portion of this research used resources at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under contract no. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
