The Trp-cage mini-protein is one of the smallest systems to exhibit a stable secondary structure and fast-folding dynamics, serving as an apt model system to study transient intermediates with both experimental and computational analyses. Previous spectroscopic studies with Trp-cage have inferred a single stable intermediate on a pathway from folded to unfolded basins. To better understand Trp-cage structural folding dynamics on microsecond-time scales, researchers probed this unfolding pathway with time-resolved X-ray solution scattering, using the resources of the Advanced Photon Source (APS), a U.S. Department of Energy Office of Science user facility at DOE’s Argonne National Laboratory.
The results indicate the formation of a conformationally extended intermediate on the time scale of 1 microsecond, which undergoes complete unfolding within 5 microseconds. The researchers further investigated the structural details of the unfolding pathway at the atomic level to generate ensemble model fits to the scattering profiles. This analysis paves the way for direct benchmarking of theoretical models of protein folding ensembles produced with molecular dynamics simulations.
BACKGROUND
The structure−function relationship of three-dimensional proteins has been a central area of scientific research for decades. Physiologically relevant protein structures have more recently been demonstrated to exhibit surprising heterogeneity, including intrinsically disordered proteins (IDP), comprising of dynamic states and flexible regions where functionality requires not just a single three-dimensional structure but an ensemble of structures. Resolving these conformational ensembles is a major challenge that would reveal insights in IDP folding/unfolding mechanisms and the functional roles of disordered intermediates.
External perturbations, such as temperature, pH, and ion concentration changes, play key roles in governing protein structural dynamics. Studying these stimuli-driven protein structural dynamics is paramount in revealing functional mechanisms. The connections of non-native structures and their dynamics are also essential for preventing diseases and guiding the discovery of new treatments.
One notable IDP model system is the 20-residue synthetic peptide TC5b, which adopts the “Trp-cage” structural motif. This Trp-cage mini-protein contains a dense web of long-range tertiary contacts around a hydrophobic core surrounding, giving it an unusually high contact order for its relatively short peptide sequence.
Previous unfolding thermodynamics studies of the Trp-cage mini-protein have demonstrated a two-state model across a broad range of temperatures. However, the debate about its folding mechanism and lack of consensus from experimental work have made Trp-cage a popular model system for molecular dynamics (MD) simulation studies.
Few experimental methods can track protein folding dynamics on time scales shorter than milliseconds and offer relatively low structural resolution. For instance, temperature-jump (T-jump) time-resolved vibrational spectroscopy methods observe the transient conformations of specific amines and carbonyls, yet concrete global information is lacking.
An emerging technique, time-resolved X-ray solution scattering (TRXSS), has been shown to capture protein folding dynamics on nanosecond to milliseconds time scales while avoiding spectroscopic probes or biochemical modifications. In this work, researchers from Northwestern University and The University of Chicago implemented temperature-jump (T-jump) TRXSS at Argonne to capture the transient conformational states of Trp-cage.
THE SCIENCE
The researchers first used small-angle X-ray solution scattering (SAXS) profiles across a range of temperatures to confirm broad temperature-induced unfolding of Trp-cage, in alignment with previous spectroscopic studies. From singular value decomposition analysis of this temperature series, only two significant intermediate species were apparent.
The researchers then performed T-jump TRXSS experiments at the BioCARS 14-ID-B beamline at the APS. The experiment was conducted from an initial temperature of 30 °C, where the temperature-dependent unfolding was induced by an infrared laser pulse, resulting in a 13 °C jump in temperature. TRXSS difference signals were obtained by subtracting the scattering patterns obtained at the two temperatures.
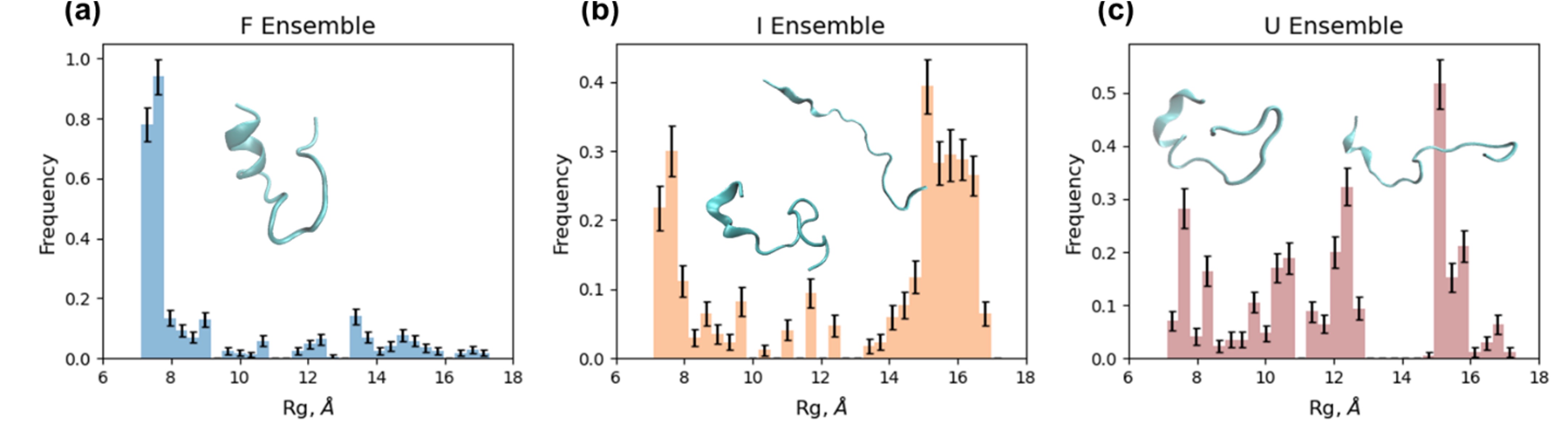
The resulting protein-associated TRXSS time series revealed the population of an unfolding intermediate species by 300 ns. SAXS experiments confirmed an unfolding structural trend within 10 microseconds of the temperature jump. The time constant of the transition between intermediate and unfolded states was determined from the fitting to be 1.2 ± 0.2 microseconds. Furthermore, ensemble modeling showed the unfolding pathway to progress through a molten globule state intermediate (Fig. 1).
The results from this work demonstrate the strengths of revealing heterogeneous protein folding dynamics through combined X-ray solution scattering and molecular dynamics simulation methods. – Chris Palmer
_____________________________________________________________________________________________
See: A. M. Chan1,, A.K. Nijhawan1, D. J. Hsu1, D. Leshchev1, D. Rimmerman1, I. Kosheleva2, K. L. Kohlstedt1, L. X. Chen1,3, “The Role of Transient Intermediate Structures in the Unfolding of the Trp-Cage Fast-Folding Protein: Generating Ensembles from Time-Resolved X-ray Solution Scattering with Genetic Algorithms,” Journal of Physical Chemistry Letters, 2023, 14, 5, 1133–1139 (January 27, 2023)
Author affiliations: 1Northwestern University; 2University of Chicago, 3Argonne National Laboratory
This work was supported by the National Institute of Health (NIH), under Contract No. R01-GM115761. A.M.C. and D.J.H. acknowledge support from the NIH/National Institute of General Medical Sciences (NIGMS) sponsored Molecular Biophysics Training Program at Northwestern University (T32GM140995). This research used resources of the APS, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of BioCARS was also supported by the NIH-NIGMS under grant number P41 GM118217.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
