If the past few years of global pandemic have taught us anything, it is that understanding viruses is of critical importance to our health. The COVID-19 pandemic was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a member of a broader family of RNA viruses that, with a few notable exceptions, had previously been known to cause mild to moderate respiratory disease in humans. The successful development of vaccines and treatments for SARS-CoV-2 was supported by research conducted during previous outbreak events, such as the fight against HIV during the 1980’s and 1990’s, the SARS-CoV-1 outbreak in 2002, and the Middle Eastern respiratory syndrome coronavirus (MERS) outbreak in 2012.
Scientists applied this same approach to studying SARS-CoV-2 in order to manage the pandemic and prepare for the next viral pandemic challenge. In a collaborative project that involved small angle X-ray scattering (SAXS) experiments conducted at the BioCAT 18-ID beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, researchers focused on how SARS-CoV-2 makes functional viral proteins from two large polyproteins that are encoded by its viral RNA genome. Gaining insights into the process through which viral proteases cleave polyproteins into functional protein pieces is crucial for understanding the SARS-CoV-2 infection cycle.
The study, conducted by a collaborative team of researchers from multiple labs in the United States and Ghana, relied upon previous studies that have shown that many RNA viruses use a “polyprotein strategy” for their replication. This strategy not only allows for a more compact genome, but it also regulates the activity of the virus through a precise spatial (where) and temporal (when) cleavage pattern. In addition, some crafty viruses appear to make use of this strategy to create multiprotein intermediates that have their own unique functions, separate from their individual protein components.
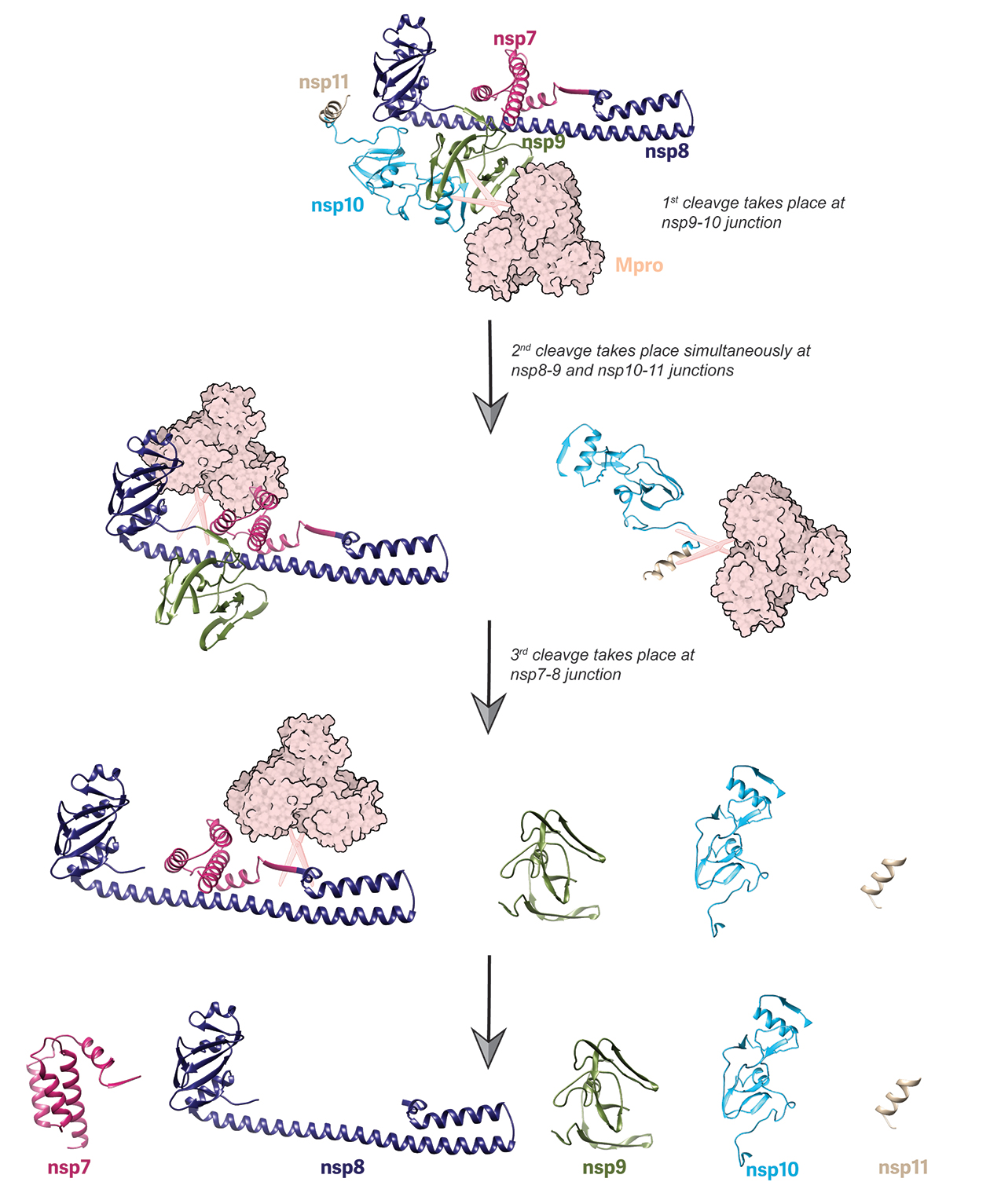
For this study, the team chose to investigate the SARS-CoV-2 nsp7-11 polyprotein that is cleaved by the main protease (called Mpro) into five nsp domains that are crucial for the full activity of the virus. After purifying the individual nsp7-11 and Mpro proteins, they incubated them together in a proteolysis assay in order to observe the sequence of polyprotein cleavage at various time points. Both SDS-PAGE gel and pulse labeling hydrogen-deuterium exchange mass spectrometry (HDX-MS) analysis confirmed that the nsp9-10 junction was cleaved first, followed by a simultaneous cleavage at the nsp8-9 and nsp10-11 junctions and, finally, the nsp7-8 junction (Fig. 1). This order, which is identical to the cleavage pattern in SARS-CoV-1, was unaffected by temperature changes or changes in concentration of any of the components.
Next, using a catalytically inactive version of Mpro, the team used continuous labeling HDX-MS and cross-linking MS (XL-MS) to show that Mpro primarily binds to the nsp9-10 junction as its initial target on the polyprotein. This is consistent with the identification of nsp9-10 as the first cleavage site of the protease. The data also showed that the interactions of Mpro and the nsp7-11 polyprotein do not induce any long-range conformational changes in either protein, except in the nsp8 N-terminal region which appears to be stabilized by Mpro binding. The data also revealed a footprint of polyprotein binding on Mpro suggesting that the interaction between Mpro and the polyprotein is at the back of the catalytic site, near the dimeric interface of Mpro, and that there are nine interprotein crosslinks between Mpro and nsp7-11. This is important information for future plans to disrupt these interactions.
Next, the team learned that nsp7-11 likely exists as both a monomer and a dimer in solution from size exclusion chromatography (SEC) coupled to multi-angle light scattering (MALS) and SAXS techniques. When these data were combined with other experimental constraints from HDX-MS and XL-MS in an integrative structural modeling approach, the team arrived at four major conformations for nsp7-11 that best fit the available data. Interestingly, two groups of conformations from the proposed ten models (see video) fit well with the cleavage sequence that the team had observed experimentally, suggesting that the nsp9-10 junction, as the initial target, was structurally the most available for cleavage with an open, random coil structure while nsp7-8, as the final cleavage site, was the most hindered structurally, adopting a helical structure in most of the models.
Finally, testing of small molecule binders of Mpro in the cleavage assay yielded more information about how these molecules may be explored in future work to identify drug candidates that block polyprotein cleavage and, potentially, SARS-CoV-2 infection. – Sandy Field
See: R. Yadav1,, V. Courouble2, S. K. Dey1, J.J. E. K. Harrison3, J. Timm1, J. B. Hopkins4, R. L. Slack5, S. G. Sarafianos5, 6, F. X. Ruiz1, P. R. Griffin2, 7, E. Arnold1, “Biochemical and Structural insights into SARS-CoV-2 Polyprotein Processing by Mpro,” Science Advances Vol. 8 Issue 49 (December 2022)
Author affiliations: 1Rutgers University; 2Scripps Research Institute; 3University of Ghana; 4Illinois Institute of Technology; 5Emory School of Medicine; 6Children’s Healthcare of Atlanta; 7University of Florida
We are grateful for support from National Institutes of Health (NIH) grants U54 AI150472 (to E.A. and P.R.G.), AI 027690 (to E.A.), F31 DK126394 (to V.V.C.), R01 AI167356 (to S.G.S.), and T32 AI157855 (to R.L.S.) and a research award from the Rutgers Center for COVID-19 Response and Pandemic Preparedness (to E.A.). We are grateful to V. Nanda and P. Falkowski for providing financial support for J.T.’s efforts. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This project was supported by grant P30 GM138395 from the National Institute of General Medical Sciences of the NIH. Use of the Pilatus 3 1M detector was provided by grant 1S10OD018090-01 from NIGMS. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of General Medical Sciences or the NIH.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
