Malaria is a serious, sometimes fatal disease caused by Plasmodium parasites. Plasmodium infection from the bite of an infected Anopheles mosquito causes people to get very sick, with fevers, chills, and flu-like illness. In 2020, there were 241 million malaria cases worldwide and 627,000 deaths, mainly among children in Africa. The seriousness of the disease has led to efforts to generate an effective vaccine, but the parasite’s ability to modify the surface proteins targeted by conventional vaccines that aim to reduce infection or morbidity has raised concerns that it might evade immune detection, rendering this strategy insufficient.
One innovative approach to overcome immune evasion is to try to block transmission of the parasite from humans to mosquitoes, thus blocking the spread of the infection. Recent work from a collaborative team of researchers from the United States, Canada, The Netherlands, and Uganda has taken this idea to the next step by identifying transmission-blocking antibodies in people who were naturally exposed to malaria parasites and mapping the structural basis for their activity. The work provides the foundation for the design of transmission-blocking vaccines against this deadly and intractable disease.
The research was based on a previous finding that one of the Plasmodium surface proteins, Pfs48/45, is important for a key reproductive step in the parasite. Plasmodium has a complex life cycle within both humans and mosquitoes, and without Pfs48/45, the parasite can’t effectively produce the infectious form that is passed to humans when the mosquito bites. Importantly, antibodies against Pfs48/45 have also been shown to prevent formation of the infectious form of the parasite, suggesting that a vaccine against Pfs48/45 could block this step in transmission. However, the generation of an appropriate Pfs48/45 vaccine has been slowed by difficulties with producing a properly folded protein to use as an antigen for vaccination.
The breakthrough from this research team was their approach of identifying transmission-blocking antibodies from human donors that targeted Pfs48/45 and then identifying the piece of the protein that elicited the response to provide a smaller, more structurally manageable antigenic target.
The first step was to identify two donors, a 69-year-old Dutch person who had spent a lot of time in Africa and had been infected many times with Plasmodium and an 8-year-old Ugandan child who had also been naturally exposed to the parasite. Antibodies in the plasma from the blood of these donors recognized Pfs48/45 and reduced parasite transmission in laboratory assays. The team isolated antibody-generating cells from the donors’ plasma and generated 81 antibodies that could bind to Pfs48/45. When they assessed the Pfs48/45 binding sites of these antibodies, they found that the antibodies bound to different regions of the protein, which contains three domains called D1, D2, and D3. In their transmission assay, the researchers showed that antibodies targeting D1 and D3 had the most potent transmission-blocking activity, while those bound to D2 had weaker activity.
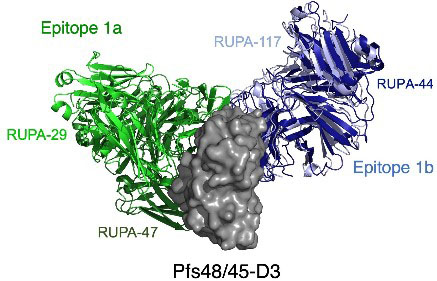
After identifying the antibodies that demonstrated the most potent transmission-blocking activity – 27 of the initial 81 antibodies could block transmission by more than 80% – they chose a subset of the antibodies that targeted D3 for further structural analysis at the GM/CA 23-ID beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory. The crystal structures of D3 in complex with the antigen binding fragments of four potent antibodies showed that the antibodies bound to two well-conserved regions of D3 that haven’t changed in variants of Plasmodium which have been isolated worldwide. This is good news because it means that these sections of the protein don’t change much over time, making them a good region of the protein to use for a long-acting vaccine that will be hard for the parasite to evade.
This work offers two potential pathways to immunization against Plasmodium parasites. First, the portion of D3 that is targeted by the antibodies is a good candidate for use as a vaccine that will enable people to generate a strong transmission-blocking antibody response and second, the antibodies they have identified and characterized can be used for passive immunization by administering them to people directly. – Sandy Field
See: A. Fabra-Garcia1,, S. Hailemariam2, R. M. de Jong1, K. Janssen1, K. Teelen1, M. van de Vegte-Bolmer1, G-J. van Gemert1, D. Ivanochko2, A. Semesi2, B. McLeod2, 3, M. W. Vos4, M. H. C. de Bruijni4, J. M. Bolscher4,, M. Szabat5, S. Vogt5, L. Kraft5, S. Duncan5, M. R. Kamya6, M. E. Feeney7, P. Jagannathan8, B. Greenhouse7, K. J. Dechering4, R. W. Sauerwein4, C. R. King9, R. S. MacGill9, T. Bousema1,, J-P. Julien2, 3,, M. M. Jore1,, “Highly Potent Naturally Acquired Human Monoclonal Antibodies Against Pfs48/45 Block Plasmodium Falciparum Transmission to Mosquitoes,” Immunity Vol. 56 Issue 2, 406-419 E7 (February 2023)
Author affiliations: 1Radboudumc; 2Hospital for Sick Children Research Institute; 3University of Toronto; 4TropIQ Health Services; 5AbCellera Biologics Inc.; 6Infectious Diseases Research Collaboration; 7University of California San Francisco; 8Stanford University; 9PATH’s Malaria Vaccine Institute
This work was funded by PATH's Malaria Vaccine Initiative and the Bill & Melinda Gates Foundation (grant no. OPP1170236). T.B. is supported by the European Research Council (ERC-CoG 864180; QUANTUM). M.M.J. is supported by the Netherlands Organisation for Scientific Research (Vidi fellowship NWO project number 192.061). This work was further supported by the CIFAR Azrieli Global Scholar program (J.-P.J.), the Ontario Early Researcher Award program (J.P.J.), the Canada Research Chair program (J.P.J.), and the Canadian Institutes of Health Research project grant #428410 (J.P.J.). S.H. is supported by an Ontario Graduate Scholarship Program and a University of Toronto Biochemistry fellowship. The BLI instrument was accessed at the Structural and Biophysical Core Facility, The Hospital for Sick Children, supported by the Canada Foundation for Innovation and Ontario Research Fund. X-ray diffraction experiments were performed at GM/CA@APS, which has been funded in whole or in part with federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006, P30GM138396). The Eiger 16M detector was funded by an NIH-Office of Research Infrastructure Programs High-End Instrumentation grant (1S10OD012289-01A1). This research used resources from the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract DE-AC02-06CH11357. Crystal structures are accessible from the Protein Data Bank under PDB: 7UNB and 7UXL. Fieldwork leading to the identification of the Ugandan donor was supported by the National Institute of Allergy and Infectious Diseases (NIAID) as part of the International Centers of Excellence in Malaria Research (ICEMR) program (U19AI089674) with laboratory support from NIAID (R01AI093615 and K24AI113002, M.E.F.).
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
