Lithium-ion batteries (LIBs) are one of the technological wonders of our time, because their high-capacity rechargeable qualities make so many of our other technological wonders possible and practical, such as cellphones, laptops, and electric vehicles. But like all devices, LIBs also have some downsides. Aside from the effort to keep pace with ever-increasing demands for longer cycling time and greater energy density, LIBs must deal with a vital safety issue: thermal instability. They can dangerously overheat under certain operating conditions, even causing fires. A team of investigators used the U.S. Department of Energy’s Advanced Photon Source (APS) to study a largely overlooked solution to this problem, involving the precise tailoring of grain microstructure in cathode materials. Their work appeared in Nature Communications.
The team focused on the nickel (Ni)-rich polycrystalline ternary material NMC Li(Ni,Mn,Co)O2, considered one of the most promising cathode materials. Using various tools, including scanning electron microscopy, neutron diffraction, and x-ray diffraction at Stanford, Brookhaven, Oak Ridge, and beamline 11-ID-C of the Advanced Photon Source, they examined in situ how the thermal stability of charged NMC cathodes degraded under heating between 25° C and 250° C, both in powder form and in delithiated electrode form in charged coin cells.
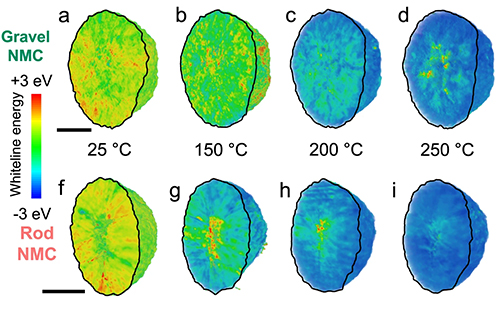
The secondary particles of the NMC cathode material are composed of primary particles in either a rod-shaped (rod-NMC) or gravel-shaped (gravel-NMC) form, which differ little in their general chemical and crystallographic characteristics. Upon charging and thermal stress, however, definite chemical and morphological differences emerge.
A range of x-ray studies were carried out using DOE user facilities. In situ heating using full-field transition x-ray microscopy was carried out at the National Synchrotron Light Source II (NSLS-II) beamline 18-ID at Brookhaven National Laboratory. X-ray absorption near edge structure measurements (Fig. 1) were performed on the electrodes in transmission mode at the APS X-ray Science Division (XSD) Spectroscopy Group’s beamline 20-BM-B of the APS at Argonne National Laboratory. Powder x-ray diffraction (XRD) was performed at the XSD Structural Science Group’s beamline 11-ID-C at the APS. Electrode XRD was performed at beamline 11-3 of Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC National Accelerator Laboratory. Together, these studies showed that Ni is the major redox-active element in both gravel-NMC and rod-NMC. Both types display similar state of charge (SoC) at the minimum temperature of 25° C and the maximum of 250° C, but rod-NMC shows faster Ni reduction and oxygen release at lower temperature ranges during ramping up, while both processes are smooth and continuous for gravel-NMC. The investigators attribute this to the chemical instability of rod-NMC. The different grain configurations result in significant differences in oxygen state evolution.
In situ time-of-flight neutron diffraction research using the Nanoscale Ordered Materials Diffractometer BL-1B instrument at the Spallation Neutron Source at Oak Ridge National Laboratory was performed for macroscale crystallographic characterization, which showed structural changes of the NMC materials during heating. As would be expected, both grain types showed thermal expansion and microstructural strain and defect propagation with chemical delithiation. Gravel-NMC showed a relatively smooth and broad transition from layered to spinel phase, while rod-NMC displayed a more abrupt phase transition, possibly accompanied by a burst of oxygen release. This, along with significant changes in grain size and shape, indicates that the rod-NMC is less stable on the macroscale than gravel-NMC due to these structural changes under thermal conditions.
In situ morphological studies also revealed severe structural instabilities in the rod-NMC material under heating. Both particle types showed hollowing of the interior upon heating, but rod-NMC showed a larger degree of collapse and particle shrinkage, leading to an overall structural degradation and possible fracturing and a markedly decreased performance at high temperatures.
The various studies on these two forms of NMC electrodes in this series of experiments provides strong evidence of the definite correlation between thermal stability and grain microstructure in LIB materials and the ways in which that correlation can contribute to battery performance, lifetime, and hazardous behavior, especially under less than optimal non-ambient operating conditions. Although the perfect cathode material for every conceivable operating parameter is certainly an unattainable ideal and certain design compromises are inevitable, this work proves that a stronger focus on grain engineering in Ni-rich polycrystalline cathodes can provide definite advantages, which can also be tailored for specific desired requirements and thermal environments, especially in combination with other design strategies. As LIBs continue to become ever more ubiquitous in our lives and ever more crucial for our daily technological well-being, such an approach can also offer greater safety and a gentler environmental impact. ― Mark Wolverton
See: Dong Hou1, Zhengrui Xu1, Zhijie Yang1, Chunguang Kuai1, Zhijia Du2, Cheng-Jun Sun3, Yang Ren3,4, Jue Liu2, Xianghui Xiao5*, and Feng Lin1**, “Effect of the grain arrangements on the thermal stability of polycrystalline nickel-rich lithium-based battery cathodes,” Nat. Commun. 13, 3437 (2022). DOI: 10.1038/s41467-022-30935-y
Author affiliations: 1Virginia Tech, 2Oak Ridge National Laboratory, 3Argonne National Laboratory, 4City University of Hong Kong, 5Brookhaven National Laboratory
Correspondence: * [email protected], ** [email protected]
The work was supported by the National Science Foundation (NSF) under Grant no. DMR-1832613 (F.L.). The National Synchrotron Light Source II is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. A portion of this research used resources at the Spallation Neutron Source, a U.S. DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. DOE Office of Science-Basic Energy Sciences under Contract No. DE-AC02-76SF00515. Some of the NMC materials were produced at the U.S. DOE’s CAMP (Cell Analysis, Modeling and Prototyping) Facility, Argonne National Laboratory. The CAMP Facility is fully supported by the DOE Vehicle Technologies Program (VTP) within the core funding of the Applied Battery Research (ABR) for Transportation Program. This work used shared facilities at the Virginia Tech National Center for Earth and Environmental Nanotechnology Infrastructure (NanoEarth), a member of the National Nanotechnology Coordinated Infrastructure (NNCI), supported by NSF (ECCS 1542100 and ECCS 2025151).
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
