Scientists have shown that advances in the synchrotron coherent x-ray scattering technique allow for probing the dynamics of live viruses in an aqueous environment that closely mimics their biological surroundings, providing real-time information of their motions at a length scale similar to the virus size. The researchers say that in the future, brighter x-rays will significantly improve the efficiency and resolution of these measurements, and allow for more accurate assessment of the potential of virus-like-particles (VLP) as vaccines or drug delivery vehicles. The results, based on research at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS), were published in the Journal of Synchrotron Radiation.
The diffusive dynamics of viruses is very similar to those of nanoparticles. Unlike certain bacteria, which can propel themselves with a flagellum, viruses are incapable of self-propelling, and their motions in water are completely determined by the continuous bombardment of surrounding water molecules from all directions. This leads to a random, microscopic movement called Brownian motion. The speed of this diffusion is determined by the virus size – the impact of being hit by water molecules is more pronounced on smaller viruses and they move faster. But the diffusion is also influenced by other factors, such as the interaction (e.g. attraction or repulsion) between the viruses and/or other solvent molecules. For instance, the protein shell of viruses consists of amino acids with charged terminals, giving viruses static surface charges that repel each other. This slows down the movement of the viruses and makes them behave as if they have a larger radius than one would expect from a neutral nanoparticle.
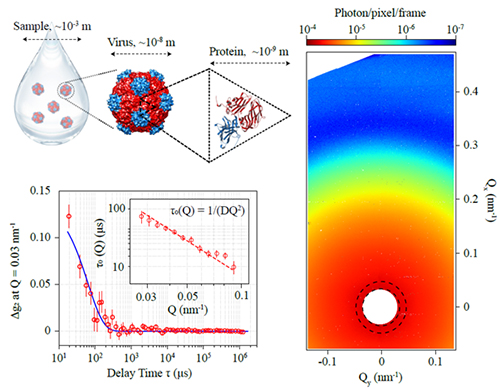
The statistics of the diffusive motion of the viruses, e.g., diffusion coefficient, can be measured using light-scattering techniques. In dynamic light scattering (DLS, also known as photon correlation spectroscopy, PCS), a laser in the visible light spectrum (wavelength 300 nm ~ 700 nm) shines on a colloidal suspension of the viruses in water or other transparent solvent. As the laser beam passes through the sample, the coherent photons from the laser scatter from each virus, and the optical interference from the scattered photons creates an intensity contrast pattern behind the sample, a.k.a., “speckle” pattern. The dynamics of the virus is evaluated via the temporal decorrelation of the speckles. Specifically, for Brownian motion, the diffusion coefficient D can be measured via the Einstein-Stokes equation D = 1/τQ2, where τ is the characteristic time of the temporal decay of the speckle intensity, and Q is the moment transfer determined by Q = 4π/λ * sin θ, where λ is the wavelength of the light and 2θ is the scatter angle at which the DLS was measured.
DLS has a few limitations. First of all, since the scattered photons are collected from the laser beam transmitting the sample, DLS cannot probe samples that are optically opaque. Secondly, the spatial sensitivity of DLS is set by the wavelength of the laser and is approximately ten times larger than a typical virus size. As a result, DLS usually only probes Brownian motion and the dynamics of the virus at the length scale of the virus size is typically extrapolated assuming the Einstein-Stokes equation. If the system becomes more complex, for example if antibodies in the solution bind to the viruses and cause them to aggregate, these assumptions no longer stand and the conclusions from the dynamic light scattering measurements are no longer valid.
To directly measure the diffusion of viruses at the scale of virus size, these researchers turned to a newer technique known as x-ray photon correlation spectroscopy (XPCS), the x-ray version of DLS that utilizes the ultra-bright, laser-like x-ray beam at the APS, a Department of Energy Office of Science user facility at Argonne National Laboratory. They carried out the XPCS measurements on a cowpea mosaic virus colloidal suspension at the X-ray Science Division Dynamics & Structure Group’s beamline 8-ID-I of the APS.
The hard x-rays used in XPCS at 8-ID-I have a wavelength of 0.11 nm, around 500 times shorter than the wavelengths of visible light used in DLS. This enabled the researchers to directly measure the motion of the virus at much smaller spatial scales and much wider spatial ranges thanks to the use of XSPA-500k, the 52-kHz frame rate, half-million-pixel array single-photon-counting detector from Rigaku Corporation.
The team found that the cowpea mosaic virus behaved as if it was 43% larger than its core size in a potassium phosphate (K-phos) buffer solution. Upon increasing the ionic strength by adding more electrolytes (e.g., sodium chloride, ammonia disulphates), the virus diffusion slowed down, which likely can be attributed to minor aggregations or slight alteration of the structure of the protein shell.
Because viruses are mostly made of lighter elements (carbon, nitrogen, oxygen, hydrogen) and do not have solid centers unlike engineered nanoparticles (e.g. LUDOX), the x-ray scattering intensity from viruses is typically hundreds of times weaker than similar-sized nanoparticles consisting of solid, heavier materials such as silica or gold. As a result, each sample condition requires averaging from ~10,000 repeating measurements resulting in 15 hours of measurement time, which prohibits investigation of complicated processes whose dynamics keeps evolving over time, such as aggregation of viruses by adding antibody molecules.
However, with the 100-times increase of coherent x-ray flux that will be provided by the upgraded APS (APS-U) that is projected to be complete in April 2024, the number of repeating measurements will reduce consequentially by 10,000 times, enabling quantitative analysis of virus dynamics in a single 4-second measurement instead of 15 hours. The new capacity of APS-U XPCS, therefore, will allow scientists to study the real-time evolution of non-Brownian virus dynamics in complex bio-relevant environments, which may lead to development of novel antiviral medications. In addition, since the protein surface of the virus can be genetically modified to mimic other viruses or selectively bind to specific bio-surfaces, and the core of the viruses can be biochemically removed or replaced with loadings that fulfil certain pharmaceutical purposes, the significantly improved measurement efficiency of APS-U XPCS will play a pivotal role in the high-throughput, guided design of biomacromolecules for use of vaccines or targeted drug delivery.
See: Kacper Switalski1, Jingyu Fan2, Luxi Li3, Miaoqi Chu3, Erik Sarnello4, Pete Jemian3, Tao Li4, Qian Wang2 and Qingteng Zhang3*, “Direct measurement of Stokes–Einstein diffusion ofCowpea mosaic virus with 19 ms-resolved XPCS,” J. Synchrotron Rad. 29, 1429 (2022). DOI: 10.1107/S1600577522008402
Author affiliations: 1University of Illinois at Chicago, 2University of South Carolina, 3Argonne National Laboratory, 4Northern Illinois University
Correspondence: * [email protected]
This work was supported in part by the U.S. Department of Energy (DOE) Office of Science, Office of Workforce Development for Teachers and Scientists (WDTS) under the Science Undergraduate Laboratory Internships Program (SULI). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy’s APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, and electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
