β-lactam-based antibiotics currently account for about 65% of all applied antibiotics, due to their broad-spectrum of activity and favorable safety profile, making this class of drugs the most common clinical approach for treating bacterial infections. Examples of these drugs, which contain a β-lactam ring in their structure, include naturally occurring penicillins, and synthetic cephalosporins, monobactams, and carbapenems. Antibiotics with a β-lactam core target bacterial transpeptidases—enzymes necessary for cell-wall synthesis—and they block the formation of cross-bridges between adjacent peptidoglycan chains, leading to bacterial death. Overuse of β-lactam antibiotics has led to an increase in microorganisms with multidrug resistance. In β-lactam antibiotics, this resistance is driven primarily by bacterial enzymes called b-lactamases. Researchers have now revealed the crystal structure, binding, and cleavage of moxalactam antibiotic bound to L1 metallo-β-lactamase (MBL) from the emerging pathogen Stenotrophomonas maltophilia using the U.S. Department of Energy’s Advanced Photon Source (APS). Drug discovery based on the details captured in this study could contribute key information to counteract antimicrobial resistance and provide tools in future pandemics. The results were published in the journal Nature Communications.
The global use of β-lactam antibiotics has resulted in many microorganisms developing drug resistance, which represents a major threat to human health and wellbeing. New treatments for multidrug-resistant and extensively drug-resistant pathogens are urgently needed. Resistance is driven primarily by the breakdown of β-lactam antibiotic structures by enzymes produced within bacteria called b-lactamases. These enzymes, which come in four classes (A, B, C, and D), are found even in bacteria that have not been exposed to synthetic antibiotics.
The most important of these resistance enzymes, called metallo-β-lactamases (MBL), belong to class B. MBLs have a large, open binding site consisting of multiple flexible loops and a di-nuclear Zn2+ scaffold that anchors the β-lactam ring in place. MBLs from B1 and B3 subclasses can break down the structure of nearly every β-lactam containing antibiotic, including the most recently developed last-resort antibiotics called carbapenems. So far, no new treatments focused on targeting MBL have been developed.
One of the most recently scrutinized MBLs is L1 from Stenotrophomonas maltophilia, belonging to the B3 subclass. S. maltophilia is an opportunistic Gram-negative bacillus multidrug-resistant pathogen that causes infections, mainly among severely immunocompromised and debilitated hospitalized patients.
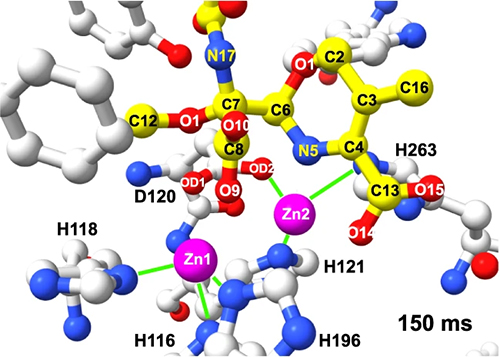
These researchers obtained serial crystallography data using the Structural Biology Center (SBC-XSD) 19-ID beamline at the APS, a Department of Energy Office of Science user facility aty Argonne National Laboratory. In addition, they employed the BioCARS 14-ID-B beamline at the APS to perform time-resolved serial synchrotron crystallography of the crystal structure, binding, and cleavage of moxalactam, a broad-spectrum, third-generation cephalosporin antibiotic bound to L1 (Fig. 1). The researchers instigated the moxalactam cleavage reaction using a 347-nm UV laser pulse to release Zn2+ ions from a UV-labile zinc-photocage in the presence of substrate. The researchers then captured 2.20-Å-resolution or better structures of L1 MBL at the following ten time points: 20, 40, 60, 80, 100, 150, 300, 500, 2,000, and 4,000 ms. These experiments and high-resolution structures revealed Zn2+ ions and substrate binding, β-lactam ring cleavage, and ligand conformational adjustments in the active site.
The researchers found that L1 adopts a typical MBL fold with two β sheets decorated with exposed α-helices and loops and that its metal scaffold is essential for recognition of the β-lactam ring. The time-lapse structures show that interaction of other moieties of the ligand with protein are secondary and explain enzyme specificity for β-lactams and their ligand promiscuity.
Interestingly, upon substrate binding the chemical transformation does not occur instantly, but instead requires ligand conformation adjustments in the active site, making the enzyme ready to attract and activate the water molecule to hydroxyl that can rapidly attack the C-8 atom. The metal scaffold, C-4, D120 carboxylate, water molecule, and nearby amino acid sidechains provide the environment to shuffle proton needed to complete the chemical transformation.
During this transformation, the distances between atoms of substrate and protein, including zinc ions change significantly, with the opening of the β-lactam ring of moxalactam occurring between C-8 and N-5 atoms. Computational modeling of the cleavage reaction shows that β-lactam ring hydrolysis was completed within 2,000 ms.
These experimental observations can aid the development of more effective inhibitors against emerging pathogens with MBL-based antibiotic resistance. In addition, the method of using time-resolved serial synchrotron crystallography with reaction triggered by photolysis of the UV-labile zinc-photocage could be applied to investigate other metalloproteins and can become a common technique for studying the dynamics of other metal-dependent enzymatic systems. ― Chris Palmer
See: M. Wilamowski1,2, D. A. Sherrell3, Y. Kim1,3, A. Lavens3, R. W. Henning1, K. Lazarski3, A. Shigemoto4, M. Endres1, N. Maltseva1, G. Babnigg1, S. C. Burdette4, V. Srajer1, and A. Joachimiak1,3*, “Time-resolved β-lactam cleavage by L1 metallo-β-lactamase,” Nat. Commun. 13, 7379 (2022). DOI: 10.1038/s41467-022-35029-3
Author affiliations: 1The University of Chicago, 2Jagiellonian University, 3Argonne National Laboratory, 4Worcester Polytechnic Institute
Correspondence: * [email protected]
The authors sincerely thank the members of SBC-XSD with data collection. Funding for this project was provided in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201700060C. SBC-XSD is supported by the U.S. Department of Energy (DOE) Office of Science, Office of Biological and Environmental Research under contract DE-AC02- 06CH11357. Use of BioCARS was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number P41 GM118217. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website
