Apoptosis—or “programmed cell death”—is a necessary process that helps keep organisms healthy in response to infection and environmental stress. Interfering with apoptosis can cause problems like cancer. While much is known about apoptosis, the exact steps that initiate this process are not completely understood. A study, which involved experiments performed at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS), reveals the structural basis for two ways in which a pro-apoptotic protein called BAK is activated during the initiation of apoptosis. Understanding this important process may one day aid in the design of “prodeath” drugs that can force cancer cells to undergo apoptosis, which in turn could shrink or eliminate tumors. The research results were published in the journal Nature Communications.
Apoptosis is a highly regulated process that is vital for the development and survival of biological organisms. Healthy cells contain a mix of both anti-death (anti-apoptotic) and pro-death (pro-apoptotic) proteins. At the start of apoptosis, pro-apoptotic proteins begin to outnumber anti-apoptotic proteins. Some of the pro-apoptotic proteins become activated, change their shape, and create pores in the mitochondrial membrane. A protein called cytochrome c is then released through these pores and activates apoptotic enzymes called caspases that dismantle and kill the cell.
A study by researchers at St. Jude Children’s Research Hospital and the University of Tennessee Health Sciences Center identifies new details about how BAK, one of the pro-apoptotic proteins that forms the pores in the mitochondrial membrane, is converted from a harmless protein to an agent of cell death during the initiation of apoptosis.
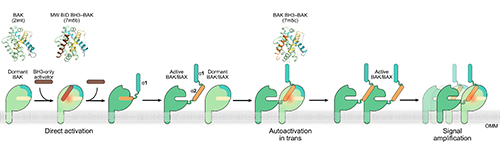
Dormant BAK can be activated into a pro-death state through direct so-called “hit and run” interactions with other activated pro-apoptotic proteins called BID and BIM at a particular site on the BAK protein (Fig. 1). However, previous studies found that BAK proteins are still able to be activated in cells lacking BID, BIM, and related proteins, suggesting another mechanism beyond this direct activation may be at play.
Biochemical evidence suggested that BAK proteins can also be “autoactivated”—meaning a dormant BAK protein could be activated by another BAK protein—but little was known about how this might occur. Using x-ray crystallography data obtained at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the APS at Argonne National Laboratory, and the FMX beamline at the National Light Source II (NSLS II) at Brookhaven National Laboratories, the study unearths the structural changes underlying BAK autoactivation. The APS and NSLS II are DOE Office of Science user facilities.
The x-ray crystallography experiments showed similarities in the three-dimensional structures of BAK proteins that were directly activated by proteins like BID and BIM and those that were autoactivated by another BAK molecule. Both cases involve contact between a helix on the interacting protein and a site called the activation groove on BAK. Binding at this site induces a conformational change that destabilizes electrostatic interactions in an alpha helix within BAK, which then initiates BAK unfolding.
The researchers also created a library of high-affinity activating and inactivating BID-like molecules, which were able to destabilize and restabilize, respectively, the alpha helix in BAK, providing further evidence that this conformational change is an important component in BAK activation. By creating a series of mutations in BAK, the researchers were able to identify which particular amino acids within BAK were needed for autoactivation and direct activation of BAK.
Finally, the team tested the role of BAK autoactivation in apoptosis. By monitoring cell death in cells where they could control which pro-apoptotic proteins were present, the researchers found that autoactivation contributes substantially to apoptosis and that the initiation of apoptosis likely involves cooperation between autoactivation and direct activation of BAK.
This study provides compelling mechanistic evidence for the structural changes that are important for both direct activation and autoactivation of BAK and for the role of BAK autoactivation in the initiation of apoptosis.
This work may inspire exploration into exciting future research avenues such as discovering how BAK autoactivation itself is initiated and determining whether BAK activation could be a target for future anti-cancer drugs. ― Summer Allen
See: Geetika Singh1,2, Cristina D. Guibao1, Jayaraman Seetharaman1, Anup Aggarwal1, Christy R. Grace1, Dan E. McNamara1, Sivaraja Vaithiyalingam1, M. Brett Waddell1, and Tudor Moldoveanu1*, “Structural basis of BAK activation in mitochondrial apoptosis initiation,” Nat. Commun. 13, 250 (2022). DOI: 10.1038/s41467-021-27851-y
Author affiliations: 1St. Jude Children’s Research Hospital, 2University of Tennessee Health Sciences Center
Correspondence: * [email protected]
Work at the FMX (17-ID-2) beamline is supported by the National Institute of Health, National Institute of General Medical Sciences (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010), and the National Synchrotron Light Source II at Brookhaven National Laboratory is supported by DOE Basic Energy Sciences under contract number DE-SC0012704 (KC0401040). SER-CAT is supported by its member institutions (see www.sercat. org/members.html), and equipment grants (S10_RR25528 and S10_RR028976) from the National Institutes of Health. This study was supported by ALSAC and NIGMS (R01GM129470) and St. Jude Cancer Center NCI (P30CA021765) funding to T.M. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.