As physicists, materials scientists, and engineers continue striving to enhance and improve batteries and other energy storage technologies, a key focus is on finding or designing new ways to make electrodes and electrolytes. One promising avenue of research involves solid-state materials, making possible batteries free of liquid electrolytes, which can pose fire and corrosion hazards. An international group of researchers joined with scientists at Argonne National Laboratory to investigate the structure of crystalline and amorphous compounds based on the NASICON system, or sodium super-ion conductors. The work (using research carried out at the U.S. Department of Energy’s Advanced Photon Source [APS] and published in the Journal of Chemical Physics) reveals some substantial differences between the crystalline and glass phases of the NAGP system, which affect the ionic conductivity of the various materials. The investigators note that the fraction of non-bridging oxygen (NBO) atoms appears to play a significant role, possibly altering the Na+ ion mobility, and suggest this as an area of further study. The work provides fresh insights into the process of homogeneous nucleation and identifying superstructural units in glass ― a necessary step in engineering effective solid-state electrolytes with enhanced ionic conductivity.
Because of their high ionic conductivity, materials with a NASICON structure are prime candidates for a solid electrolyte in sodium-ion batteries. They can be prepared by a glass-ceramic route, which involves the crystallization of a precursor glass, giving them the usefulness of moldable bulk materials. In this work, the research team specifically studied the NAGP system [Na1+xAlxGe2-x(PO4)3] with x = 0, 0.4 and 0.8 in both crystalline and glassy forms. Working at several different facilities, they used a combination of techniques, including neutron and x-ray diffraction, along with 27Al and 31P magic angle spinning and 31P/23Na double-resonance nuclear magnetic resonance spectroscopy. The glassy form of NAGP materials was examined both in its as-prepared state and after thermal annealing, so that the changes on crystal nucleation could be studied.
Neutron powder diffraction measurements were performed at the BER II reactor source, Helmholtz-Zentrum Berlin, using the fine resolution powder diffractometer E9 (FIREPOD), followed by Rietveld analysis. Further neutron diffraction observations were conducted at the Institut Laue-Langevin using the D4c diffractometer and at the ISIS pulsed neutron source using the GEM diffractometer. X-ray diffraction studies were performed at X-ray Science Division Magnetic Materials Group’s beamline 6-ID-D of the APS, an Office of Science user facility at Argonne National Laboratory.
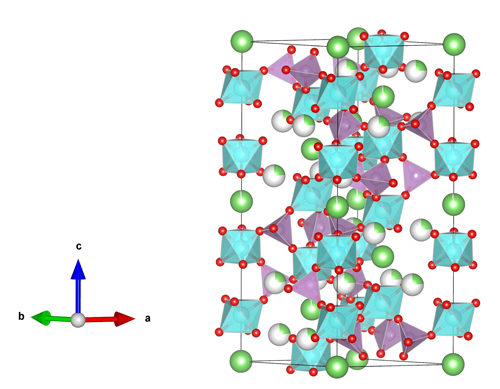
The studies reveal the structural changes that accompany the increase with x in the ionic conductivity of the crystalline material (Fig. 1). The NAGP x = 0 and x = 0.4 compounds are classified as space group R3 while the x = 0.8 compound is space group R3c. The x = 0 phase shows tetrahedral PO4 motifs linked by bridging oxygen (BO) atoms to four octahedral GeO6 motifs. This permits Na+ ions to reside at the interstices, allowing ionic conductivity. In the glassy NAGP phases, the formation of sub-octahedral Ge and Al-centered units leads to NBO atoms. Upon annealing, the fraction of NBO atoms decreases as the Ge and Al coordination numbers increase. Again, the ionic conductivity increases with the concentration of Na+ ions in the glassy NAGP material.
Based on the Ren and Eckert model for vitreous sodium phosphosilicates, the researchers propose a model for the x = 0 glass in which superstructural units are formed. In these units, P(3) phosphate motifs with three BO and one NBO atoms are converted to P(4) phosphate motifs with four BO atoms, thereby converting GeO4 to GeO6 motifs and increasing the size of the superstructural units. Annealing the as-prepared glass leads to increases in both the Ge coordination number and the fraction of P(4) motifs, which provide the nucleation sites for crystal growth. ― Mark Wolverton
See: Lawrence V. D. Gammond1, Henry Auer2, Rita Mendes Da Silva1, Anita Zeidler1, Jairo F. Ortiz-Mosquera3, Adriana M. Nieto-Muñoz3, Ana Candida M. Rodrigues3, Igor d'Anciães Almeida Silva4, Hellmut Eckert4, 5, Chris J. Benmore6, and Philip S. Salmon1*, “Structure of crystalline and amorphous materials in the NASICON system Na1+xAlxGe2―x(PO4)3,” J. Chem. Phys. 155, 074501 (2021). DOI: 10.1063/5.0049399
Author affiliations: 1University of Bath, 2Fraunhofer Institute for Ceramic Technologies and Systems IKTS, 3Universidade Federal de São Carlos, 4Universidade de São Paulo, 5Institut für Physikalische Chemie, 6Argonne National Laboratory
Correspondence: * [email protected]
LVDG acknowledges funding and support from the EPSRC Centre for Doctoral Training in Condensed Matter Physics (CDT-CMP), Grant No. EP/L015544/1, the Science and Technology Facilities Council (STFC) and Diamond Light Source Ltd (Reference No. STU0173). RMDS acknowledges funding and support from the Royal Society. PSS received support from the University of Bath's International Funding Scheme. AZ was supported by a Royal Society-EPSRC Dorothy Hodgkin Research Fellowship. IDAS and HE appreciate funding by FAPESP, Center of Research, Technology and Education, process number 2013/07793-6. IDAS also acknowledges FAPESP funding for a postdoctoral fellowship, process number 2017/17800-0. JFOM and AMNM were supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Process Nos. 168682/2017-6 and 141220/2016-3, respectively. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
