To understand how proteins participate in physiological processes, scientists probe crystallized proteins using x-ray diffraction to determine their structures and how those structures change during physiological activities. However, proteins are fragile (compared with other carbon-based materials) and data collection conditions for investigating them don't always correspond to the protein's physiological temperature. A team of collaborators made changes to the basic diffractometer design to address these issues and using the U.S. Department of Energy’s Advanced Photon Source (APS) demonstrated how their new diffractometer platform automatically conducts large-scale, serial Laue diffraction at room temperature directly from the devices where the crystals were grown. Their results were published in IUCrJ.
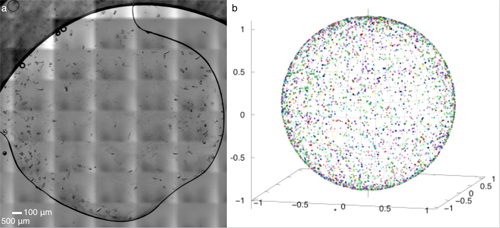
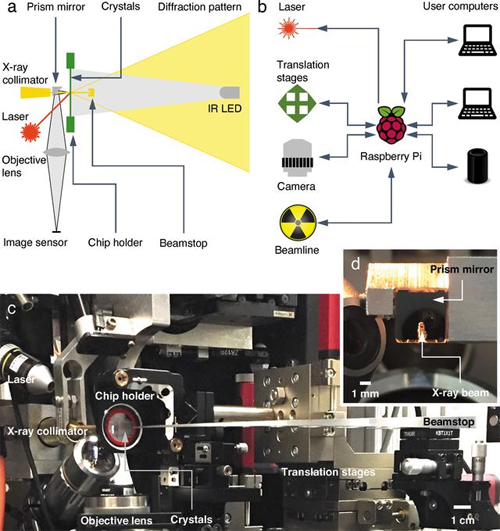
There are two major hurdles to successful collection of structural information about proteins using x-rays. One is the difficulty in collecting data in an environment which allows scientists to watch the protein's physiological activities occur in crystals. Protein crystallography is commonly performed at below-freezing temperatures (called cryocrystallography) because x-ray radiation at room temperature damages the protein crystals quickly. The second is the complicated procedures to transfer, mount, load, and collect data on protein crystals without exposing them to physical stress or drying them out. To overcome these hurdles, the team of researchers from the University of Illinois at Chicago, Renz Research, Inc., and the University of Maryland developed an automated diffractometer platform with additional capabilities which collects monochromatic and Laue diffraction data of large arrays of protein crystals (Fig. 2). The platform minimizes the radiation damage to the crystals by requiring only a single x-ray exposure for each crystal. The platform collects data on large arrays of in situ protein crystals―crystals which are assessed in the same chamber where they were grown―allowing fragile proteins to be studied more easily without requiring sample manipulation. The platform includes hardware for holding the samples, the ability to adjust the position of the sample array for use with different sources and different facilities, and a Raspberry Pi computer, which interfaces between the diffractometer hardware and the computers used to control and collect data from the diffractometer. Figure 1 shows an example of the protein crystals and the range of their orientations.
To demonstrate the efficacy of their platform, the team worked with two photosensitive bilin-based photoreceptors, which are representative of difficult types of proteins because they typically diffract weakly, their diffraction degrades quickly, and they are highly sensitive to visible light. The team used their previously published process to synthesize crystal-on-crystal sample arrays. They then collected Laue diffraction data at room temperature using the diffractometer platform with the BioCARS x-ray beamline 14-ID-B and the Life Sciences Collaborative Access Team x-ray beamline 21-ID-D, both at the APS, which is an Office of Science user facility at Argonne National Laboratory.
For the first protein (a far-red photoreceptor from Anabaena cylindrica), they compared the platform's results with those of previously collected cryocrystallographic data and found that the new platform provided better-resolved electron-density maps and refinement statistics. Additionally, the new platform's electron-density maps for the first protein had no gaps in the protein's backbone. For both proteins, the team also collected pump-probe data.
For the second protein (the photosensory core module of a bacteriophytochrome) they obtained a difference Fourier map which showed light-induced structural changes which were better-resolved than previously published cryocrystallographic data for the same photosensory core module.
The well-resolved structures of these fragile example proteins collected by the team at room temperature demonstrate the potential of this prototype diffractometer platform to reveal protein activities in physiologically-relevant environments, bringing us closer to better understanding of the cellular processes in which proteins participate. ― Mary Alexandra Agner.
See: Zhong Ren1,2* Cong Wang1, Heewhan Shin1, Sepalika Bandara1, Indika Kumarapperuma1, Michael Y. Ren3, Weijia Kang1, and Xiaojing Yang1**, “An automated platform for in situ serial crystallography at room temperature,” IUCrJ 7(6), 1009 (November 2020). DOI: 10.1107/S2052252520011288
Author affiliations: 1University of Illinois at Chicago, 2Renz Research, Inc., 3University of Maryland,
Correspondence: * [email protected], ** [email protected]
This work is supported by grants from the University of Illinois at Chicago, the National Institutes of Health (grant No. R01EY024363 awarded to X.Y.), and the National Science Foundation (grant No. MCB2017274 awarded to X.Y.). Use of BioCARS was supported by the National Institute of General Medical Sciences of the National Institutes of Health (grant No. P41 GM118217). Use of the Life Sciences Collaborative Access Team was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri- Corridor (grant No. 085P1000817).
See also: “Crystal structure of a far-red–sensing cyanobacteriochrome reveals an atypical bilin conformation and spectral tuning mechanism,” PNAS 118 (12), e2025094118 (March 23, 2021). DOI: 10.1073/pnas.2025094118 and APS science highlight “A Monocrystalline Device for Room-Temperature Serial Diffraction”
This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
