A class of materials known as lead halide perovskites (LHPs) show remarkable potential for use in optoelectronic applications. Given the urgency to derive more of our energy renewably, LHPs hold out great promise as materials for thin-film solar cells that are both highly efficient and inexpensive to produce. Although LHPs have been extensively studied, the exact process of how an incident photon is converted into an electrical charge and then migrates within the material has remained open to debate. To resolve this mystery, researchers performed x-ray transient absorption (XTA) on two different types of thin-film LHPs. In experiments carried out at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) a team of researchers from two DOE national laboratories team used XTA to detect the creation and movement of electrical carriers within the thin films. The experiments revealed exactly how the crystalline structure of thin-film LHP is excited by incident photons, as well as how the resulting charged regions behave within the material. The experiments also showed that the photo-generated charges behaved differently in the two LHPs. These direct observations of photo-induced charges and their movement within thin-film LHPs should improve the theoretical framework used to describe these materials, thereby hastening their practical use for solar power and other applications. The results were published in The Journal of Physical Chemistry Letters.
The efficiency of a solar cell indicates how readily it converts sunlight into useful electricity. Scientists have created LHP solar cells that achieve 25% efficiency, which is among the highest for any thin-film material. In addition to high efficiency, thin-film LHP solar cells are typically lightweight, flexible, and inexpensive to mass produce. In spite of these advantageous features, several issues have plagued the deployment of LHP solar power, most notably a pronounced degradation of the thin film when exposed to sunlight. Fortunately, dramatic improvements in the stability of LHP solar cells has recently been achieved, but additional progress is needed before these materials are ready for large-scale production. This progress includes a better understanding of how LHPs fundamentally function.
The two thin-film LHPs examined in this study were methylammonium lead bromide (CH3NH3PbBr3) and aminomethyl lead bromide (CH(NH2)2PbBr3). As these names imply, both compounds contain the elements lead (Pb) and bromine (Br). The difference between these two LHPs is their attached organic (carbon-based) molecules, which impart slightly different properties. Both compounds are crystalline semiconductors with a perovskite structure. The perovskite shape consists of six bromine atoms forming an octahedral cage (resembling two pyramids connected at their base) with a single lead atom at the center.
Semiconductors, including the LHPs studied here, typically generate electrical current through the movement of electrons and holes. A hole is a region in a semiconductor where an electron has been extracted. When a semiconductor is excited by a photon it creates electrons and holes, which are both considered charge carries. The presence of a localized charge carrier (an electron or hole) within a semiconductor distorts its lattice. This distortion, called a polaron, moves in lockstep with the charge carrier. While scientists knew that photoexcitation generates charge carriers and their associated polarons in thin-film LHPs, there was considerable uncertainty about their position and movement.
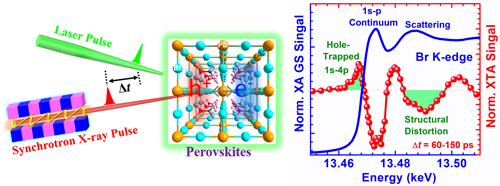
The researchers from Argonne National Laboratory and Los Alamos National Laboratory set out to resolve these uncertainties using a combination of ultrafast experimental techniques. The femtosecond and nanosecond optical transient absorption spectra of ~100-nm perovskite thin-film samples were measured at the Argonne Center for Nanoscale Materials (CNM). Each LHP sample was exposed to an extremely brief laser pulse lasting around one-tenth of a picosecond (one picosecond is a trillionth of a second). The laser pulse momentarily induced electrons and holes to appear in the thin-film sample. This was immediately followed by XTA spectroscopy using an 80-picosecond burst of x-rays at the X-ray Science Division Time Resolved Research Group’s 11-ID-D x-ray beamline at the APS (the CNM and the APS are Office of Science user facilities at Argonne). The x-rays were tuned for absorption by the sample's bromine atoms (Fig. 1). The XTA technique unequivocally demonstrated that the positively charged holes and associated polarons generated by the laser pulse arose at, and were centered on, the sample's bromine atoms, and not some other site such as the film's lead atoms.
The data also revealed a difference between the two types of LHPs examined in the study. Specifically, the holes in the thin film made of aminomethyl lead bromide (CH(NH2)2PbBr3) lasted longer before decaying and supported larger and longer lasting polarons.
These experimental results constitute the direct observation of light-induced hole and associated polaron formation in thin-film LHPs. This enhanced understanding of how charge is transported in these promising materials should lead to higher performance of LHP-based solar cells, as well as promoting LHPs for potential use in many other optoelectronic applications, including light emitting diodes (LEDs), lasers, and photodetectors. — Philip Koth
See: Cunming Liu1*, Hsinhan Tsai2, Wanyi Nie2, David Gosztola1, and Xiaoyi Zhang1**, “Direct Spectroscopic Observation of the Hole Polaron in Lead Halide Perovskites,” J. Phys. Chem. Lett. 11, 6256 (2020). DOI: 10.1021/acs.jpclett.0c01708
Author affiliations: 1Argonne National Laboratory, 2Los Alamos National Laboratory
Correspondence: * [email protected], ** [email protected]
This work was supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, under Contract DE-AC02-06CH11357. The laser system at 11-ID-D of the APS was funded through New Facility and Midscale Instrumentation grants to the Chemical Sciences and Engineering Division of Argonne National Laboratory (ANL) (PI, Lin X. Chen). W.N. acknowledges the Director’s quantum initiative funding from Laboratory Directed Research Directions (LDRD) program at Los Alamos National Laboratory (LANL). H.T. acknowledges the financial support from a Robert Oppenheimer (JRO) Distinguished Postdoc Fellowship at LANL. This work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated for the DOE Office of Science by LANL (Contract 89233218CNA000001). Use of the Center for Nanoscale Materials, an Office of Science user facility, was supported by the U.S. DOE Office of Science-Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
