Refractory materials, defined as nonmetallic substances with very high melting points that allow them to withstand extremely high temperatures above 1500° C, are essential for industrial applications and processes that occur in such extreme conditions. Refractory materials are used to coat the interiors of furnaces, incinerators, reactors, and wherever it's necessary to withstand great pressures and temperatures. Properly testing such materials in such extreme environments is obviously challenging, so materials scientists greatly depend upon computational modeling techniques to accurately characterize the behavior of refractory materials. Methods such as ab initio molecular dynamics simulations (AIMD) have their limitations, however. Some modeling techniques can only handle relatively small systems and short time scales, or do not always agree with experimental data. Recent attempts to address these issues have focused on incorporating machine learning methods with quantum-mechanical calculations to achieve models of larger systems and time scales. A group of researchers demonstrated this approach by creating a scheme to generate multiphase machine learning inter-atomic potentials (ML-IP) for the common refractory oxide material hafnium dioxide (HfO2) and testing it at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source and Spallation Neutron Source. Their results were published as an Editor’s Suggestion in Physical Review Letters.
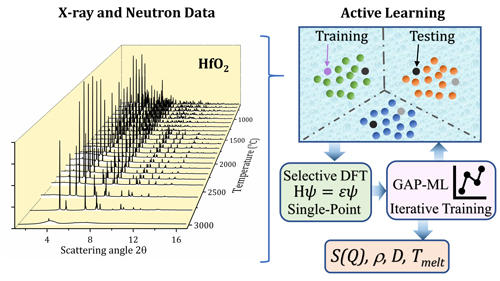
The investigators from the from University of Cambridge (UK), Helmholtz-Institute Munster (Germany), and Argonne National Laboratory devised an automated scheme of three parts, beginning with experimental measurements of sample material to obtain phase information up to the melting point. In this work, X-ray diffraction data was collected on high-purity samples of HfO2 up to about 3000° C in reducing and oxygen atmospheres at the X-ray Science Division Magnetic Materials Group’s 6-ID-D x-ray beamline of the APS, an Office of Science user facility at Argonne , followed by complementary neutron diffraction measurements at the Spallation Neutron Source of Oak Ridge National Laboratory (Fig.1).
In the second step of the researchers' automated scheme, the dataset of x-ray and neutron diffraction measurements is used to initialize active learning to create approximate ML-IP models of the interaction potentials between atoms, and then in the final step, using these to perform ab initio calculations which are used to retrain the ML-IP model to its final form. The process continues in a closed loop until an interatomic potential is found that can reproduce the measured structures over the experimentally determined phase space.
The active learning in this demonstration uses the Gaussian approximation potential (GAP) framework to generate and train the ML-IP model. ML-IP based on GAPs have been previously shown to be applicable to a broad range of models including liquids, crystal defects, and amorphous, multicomponent, and molecular systems. Here, the resulting model spans the HfO2 phase space from the liquid to amorphous to crystalline over 2053 configurations.
As it is heated, HfO2 passes from monoclinic to tetragonal to cubic phases and melts at about 2800° C. Production simulations were conducted with the ML-IP model, using a 6144-atom cell for monoclinic and cubic HfO2 and a 6912-atom cell for the tetragonal phase. The decrease in long-range ordering with higher temperatures seen by neutron diffraction patterns in the tetragonal and cubic forms in the liquid and amorphous phases was accurately shown in the ML model. Multiphase potential of HfO2 was also theoretically evaluated by comparing cohesive energy and diffusion coefficients using several methods. The GAP technique was most accurate in predicting cohesive energies. Diffusion calculations based on the MD simulations show little diffusion of either Hf or O at simulation temperatures but indicate greater diffusion of Hf in liquid HfO2 at higher temperatures.
The current work provides an effective proof of concept demonstrating how a three-step automated scheme can be used to generate a multiphase ML-IP for a refractory oxide material. The scheme can initialize ab initio calculations directly from experimental models or measurements and thus effectively validate the models with ab initio accuracy.
The scheme presented here can also be used with ML-IP techniques other than the GAP framework employed in this demonstration and can be adapted to characterize a wide range of refractory oxides and similar materials. It offers a new tool for computational modeling and simulation to supplement and enhance the development of unique materials for difficult applications. ― Mark Wolverton
See: 1Ganesh Sivaraman, 1Leighanne Gallington, 2Anand Narayanan Krishnamoorthy, 1Marius Stan, 3Gábor Csányi, 1Álvaro Vázquez-Mayagoitia, and 1Chris J. Benmore*, “Experimentally Driven Automated Machine-Learned Interatomic Potential for a Refractory Oxide,” Phys. Rev. Lett. 126, 156002 (2021). DOI: 10.1103/PhysRevLett.126.156002
Author affiliations: 1Argonne National Laboratory, 2Helmholtz-Institute Munster, 3University of Cambridge
Correspondence: * [email protected]
This material is based upon work supported by Laboratory Directed Research and Development (LDRD) funding from Argonne National Laboratory, provided by the Director, Office of Science, of the U.S. Department of Energy (DOE) under Contract No. DE-AC02-06CH11357. This research used resources of the Argonne Leadership Computing Facility, which is a DOE Office of Science User Facility supported under Contract No. DE-AC02- 06CH11357. Argonne National Laboratory’s work was supported by the U.S. DOE Office of Science, under Contract No. DE-AC02-06CH11357. We gratefully acknowledge the computing resources provided on Bebop; a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357 and the Spallation Neutron Source operated by Oak Ridge National Laboratory. Use of the Center for Nanoscale Materials, an Office of Science user facility, was supported by the U.S. DOE Office of Science-Basic Energy Sciences, under Contract No. DEAC02- 06CH11357. G. S. would like to thank A. N. K gratefully acknowledges financial support from the German Funding Agency (Deutsche Forschungsgemeinschaft-DFG) under Germany’s Excellence Strategy—EXC 2075—390740016.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
