Where cancer is concerned, sometimes the treatment can be as bad as the disease, with invasive surgeries and aggressive chemotherapies taking a great toll on the health of patients. The search for effective noninvasive cancer treatments remains an active area of research. One promising strategy that is already in use as a cancer treatment is photodynamic therapy (PDT), which involves using light in combination with a photosensitizer to generate reactive oxygen species (ROS) and kill cancer cells. The approach has been limited by current photosensitizers, which have a number of drawbacks. Researchers are developing novel photosensitizers, such as nanoscale metal-organic framework (MOF)-stabilized bacteriochlorins, to overcome these limitations. In this study, researchers from The University of Chicago characterized these new photosensitizers using the U.S. Department of Energy’s Advanced Photon Source. They also took it a step further, testing the treatment strategy in cancer cells and animal models. Their results, published in the Journal of the American Chemical Society, shine new light on PDT as an effective and safe treatment for cancer.
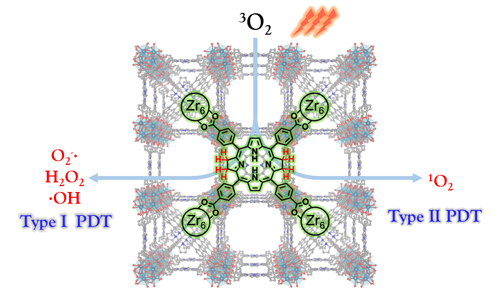
PDT requires three components: a light source, a photosensitizer, and tissue oxygen. A patient undergoing PDT is first treated with the photosensitizer, either systemically or locally. Then, once the photosensitizer inhabits the cancer cells, a light tuned to a particular wavelength is shone on the malignancy, beginning a cytotoxic molecular cascade that can go in multiple directions. In type I PDT, the light-activated photosensitizer reacts with tissue oxygen or other biomolecules to create free radicals, which then go on to damage and kill cancer cells. In type II PDT, the photosensitizer transfers energy to oxygen and releases singlet oxygen, which also wreaks havoc on cancer cells. Type II PDT is known to be dependent on tissue oxygen level.
Conventional photosensitizers for PDT absorb light in the visible range, which cannot penetrate deeply in tissues thus limiting treatment efficacy and causing photosensitivity in healthy surrounding tissues. They also require that a cancer mass contains significant amounts of oxygen, which is not always the case, as some tumors have low oxygen, limiting PDT's efficacy with conventional photosensitizers. To meet these challenges, researchers are turning to bacteriochlorin, a photosensitizer with minimal absorption in the visible range, the capacity to work in low oxygen environments, and, as an added bonus, strong absorption in the near-infrared range, which translates into a robust PDT response. However, these advantages come at a price: bacteriochlorin breaks down when exposed to oxygen and light.
To overcome bacteriochlorin's instability, researchers are using nanoscale MOFs, crystalline molecular scaffolds containing metal centers surrounded by organic ligands. In this study, bacteriochlorin was introduced into a nanoscale MOF to help stabilize the photosensitizer and enhance PDT. To solve the structure of the hafnium-based MOF (Hf-TBB), the researchers performed single-crystal x-ray diffraction with a Bruker APEX II CCD-based detector at the ChemMatCARS 15-ID x-ray beamline at the Advanced Photon Source. Zirconium, however, which is chemically similar to hafnium, became the metal of choice for a photosensitizer in this study. Powder x-ray diffraction pattern studies showed that bacteriochlorin in a zirconium-based MOF (Zr-TBB) adopted the same structure as Hf-TBB. Additional analytical studies demonstrated that Zr-TBB had superior stability in the presence of light and oxygen compared to the naked bacteriochlorin, thanks to the protection of the geometric framework.
Next, the researchers tested Zr-TBBs ability to produce cancer-killing oxygen species. They irradiated Zr-TBB in the presence of oxygen with 740-nanometer wavelength light, and found the photosensitizer produced various reactive oxygen species—superoxide anions, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. This supported Zr-TBB as an effective agent for PDT with the capacity to access both type I and type II PDT mechanisms. The researchers observed the generation of these radical species in vitro, using breast carcinoma cells, finding greatly increased cytotoxicity and immunogenic cell death in those cells treated with Zr-TBB and light.
As a final test, the researchers explored the therapeutic value of Zr-TBB in vivo. Using mouse models of breast and colon cancer, PDT treatment with Zr-TBB led to a 40% and 60% cure rates, respectively.
These findings indicate that nanoscale MOFs offer a promising platform for PDT photosensitizers, including bacteriochlorins as well as other molecules. — Erika Gebel Berg
See: Taokun Luo, Kaiyuan Ni, August Culbert, Guangxu Lan, Zhe Li, Xiaomin Jiang, Michael Kaufmann, and Wenbin Lin*, “Nanoscale Metal−Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy,” J. Am. Chem. Soc. 142, 7334 (2020). DOI: 10.1021/jacs.0c02129
Author affiliation: The University of Chicago
Correspondence: * [email protected]
We thank Yang Song, Xuanyu Feng, Yunhong Pi, and Wenbo Han for experimental help. We acknowledge the National Cancer Institute (U01-CA198989) and The University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599) for funding support. ChemMatCARS is principally supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under Grant NSF/ CHE-1834750. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
