 Middle East respiratory syndrome coronavirus (MERS-CoV) is one of five β-coronaviruses in the family Coronaviridae found to infect humans. In 2012, it emerged as a highly fatal cause of severe acute respiratory infection. Although no anti-MERS-CoV therapy is available yet, potently neutralizing antibodies targeting the receptor-binding domain (RBD) on the MERS-CoV spike glycoprotein have been characterized, but much less is known about antibodies targeting non-RBD epitopes. Researchers have now derived the molecular structure and functional characterization of G2, a neutralizing antibody targeting the N-terminal domain (S1-NTD) of the S1 subunit of the MERS-CoV spike glycoprotein. Crystal structures of G2 alone and in complex with the MERS-CoV S1-NTD obtained at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS), along with biochemical, biophysical, and cell-based assays reveal a site of vulnerability on the MERS-CoV spike glycoprotein and point to a neutralization mechanism whereby G2 inhibits the attachment of DPP4 to the spike protein. These results increase the understanding of the viral attachment mechanism and may facilitate the development of S1-NTD-based vaccines against MERS-CoV.
Middle East respiratory syndrome coronavirus (MERS-CoV) is one of five β-coronaviruses in the family Coronaviridae found to infect humans. In 2012, it emerged as a highly fatal cause of severe acute respiratory infection. Although no anti-MERS-CoV therapy is available yet, potently neutralizing antibodies targeting the receptor-binding domain (RBD) on the MERS-CoV spike glycoprotein have been characterized, but much less is known about antibodies targeting non-RBD epitopes. Researchers have now derived the molecular structure and functional characterization of G2, a neutralizing antibody targeting the N-terminal domain (S1-NTD) of the S1 subunit of the MERS-CoV spike glycoprotein. Crystal structures of G2 alone and in complex with the MERS-CoV S1-NTD obtained at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS), along with biochemical, biophysical, and cell-based assays reveal a site of vulnerability on the MERS-CoV spike glycoprotein and point to a neutralization mechanism whereby G2 inhibits the attachment of DPP4 to the spike protein. These results increase the understanding of the viral attachment mechanism and may facilitate the development of S1-NTD-based vaccines against MERS-CoV.
MERS-CoV was first identified in Saudi Arabia in June 2012. Infection by this pathogen causes an acute respiratory disease designated as MERS, with symptoms that are very similar to those of SARS. Globally, MERS-CoV infections have been confirmed in dozens of countries causing more than 800 deaths over the years, with most cases coming from the Middle East.
MERS-CoV likely originated from bats, with camels functioning as a secondary or intermediate host. However, many infected patients without bat or camel exposure demonstrate that large-scale human-to-human transmissions can occur through close contacts. Due to its potential for mutating toward efficient human-to-human transmission and causing a pandemic, MERS-CoV was listed as a Category C Priority Pathogen by the U.S. National Institute of Allergy and Infectious Diseases. The high case fatality rate, vaguely defined epidemiology, and absence of prophylactic or therapeutic measures against this novel virus raise the persistent concern about a possible pandemic and create an urgent need for an effective vaccine.
Past efforts to develop coronavirus vaccines have used whole-inactivated virus, live-attenuated virus, recombinant protein subunit or genetic approaches. The primary target for neutralizing antibodies is the Spike (S) glycoprotein, cleaved into two subunits: S1, which is distal to the virus membrane and S2, which contains both a transmembrane domain and two heptad-repeat sequences typical of class I fusion glycoproteins. The S1 subunit has been the focus of most immunization strategies against MERS-CoV, as it contains the receptor-binding domain (RBD) that mediates virus attachment to its receptor, dipeptidyl peptidase-4 (DPP4), on the membrane of host cells.
Several neutralizing antibodies targeting MERS-CoV S1-NTD have been reported, including human antibody CDC2-A2, murine antibodies G2 and 5F9, and macaque antibodies FIB-H1 and JC57-13. Of these antibodies, G2 is the most potent, with broad neutralization potential against an array of MERS-CoV strains. Additionally, G2 and other NTD-specific MERS-CoV antibodies have been shown to confer protection against lethal challenge in animal models. However, the lack of structural information for G2 has hindered the definition of its epitope and determination of its mechanism of action.
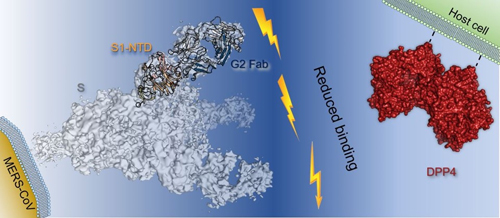
To better understand G2, researchers at the University of Texas at Austin, the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases, the Scripps Research Institute, and Vanderbilt University School of Medicine, derived the crystal structures of G2 Fab alone and bound to the MERS-CoV S1-NTD. Diffraction data were collected at the Structural Biology Center (SBC-XSD) beamline 19-ID-D at the APS, an Office of Science user facility at Argonne National Laboratory. The crystal structures define a site of vulnerability comprising two loops, each of which contain a residue mutated in G2-escape variants. Cell-surface binding studies and in vitro competition experiments demonstrate that G2 strongly disrupts the attachment of the MERS-CoV spike glycoprotein to its host receptor, dipeptidyl peptidase-4 (DPP4), with the inhibition requiring the native trimeric spike protein conformation (Fig. 1).
This study advances the understanding of antibody-mediated neutralization of coronaviruses and may inspire structure-based vaccine design to thwart MERS-CoV. ― Chris Palmer
See: Nianshuang Wang1, Osnat Rosen2‡, Lingshu Wang2, Hannah L. Turner3, Laura J. Stevens4, Kizzmekia S. Corbett2, Charles A. Bowman3, Jesper Pallesen3, Wei Shi2, Yi Zhang2, Kwanyee Leung2, Robert N. Kirchdoerfer3, Michelle M. Becker4, Mark R. Denison4, James D. Chappell4, Andrew B. Ward3, Barney S. Graham2, and Jason S. McLellan1*, “Structural Definition of a Neutralization-Sensitive Epitope on the MERS-CoV S1-NTD,” Cell Rep. 28, 3395 (September 24, 2019). DOI: 10.1016/j.celrep.2019.08.052
Author affiliations: 1The University of Texas at Austin, 2National Institute of Allergy and Infectious Diseases, 3Scripps Research Institute, 4Vanderbilt University School of Medicine, ‡Present address: Israel Institute for Biological Research
Correspondence: *[email protected]
This work was supported by National Institutes of Health (NIH) grant R01AI127521 (to J.S.M. and A.B.W.), NIH contract HHSN261200800001E agreement 16x142 (to M.R.D. and J.D.C.), and intramural funding from National Institute of Allergy and Infectious Diseases for work at the VRC (B.S.G.). SBC-XSD is operated by UChicago Argonne, LLC, for the U.S. Department of Energy (DOE) Office of Biological and Environmental Research under contract DE-AC02-06CH11357. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners.
The U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
