 The original Georgia Tech press release by John Toon can be read here.
The original Georgia Tech press release by John Toon can be read here.
Highly intense x-ray beams and a clever experimental setup allowed researchers to watch a high-pressure, high-temperature chemical reaction to determine for the first time what controls formation of two different nanoscale crystalline structures in the metal cobalt. The technique, employed at two U.S. Department of Energy x-ray light sources, including the Advanced Photon Source, allowed continuous study of cobalt nanoparticles as they grew from clusters including 10s of atoms, to crystals as large as 5 nanometers. The research provides the proof-of-principle for a new technique to study crystal formation in real time, with potential applications for other materials, including alloys and oxides.
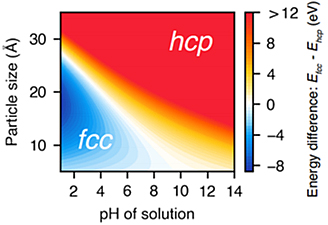
Data from the study published in the Journal of the American Chemical Society, produced “nanometric phase diagrams” showing the conditions that control the structure of cobalt nanocrystals as they form.
“We found that we could indeed control formation of the two different crystalline structures, and that the tuning factor was the pH of the solution,” said Hailong Chen, assistant professor in the George W. Woodruff School of Mechanical Engineering at the Georgia Institute of Technology and lead author of the study. “Tuning the crystalline structure allowed us to control the functionality and properties of these materials. We believe this methodology could also be applied to alloys and oxides.”
In bulk cobalt, crystal formation favors the hexagonal close-pack structure because it minimizes energy to create a stable structure. At the nanoscale, however, cobalt also forms the face-centered cubic phase, which has a higher energy. That can be stable because the high surface energy of small nanoclusters affects the total crystalline energy, Chen said.
“When the clusters are small, we have more tuning effects, which is controlled by the surface energy of the OH minus group or other ligands,” he added. “We can tune the concentration of the OH minus group in the solution so we can tune the surface energy and therefore the overall energy of the cluster.”
Working with researchers from Argonne National Laboratory and Brookhaven National Laboratory, and from the University of Maryland, Chen and graduate research assistant Xuetian Ma examined the polymorphic structures using theoretical, experimental and computational modeling techniques.
Experimentally, the researchers reduced cobalt hydroxide in a solution of ethylene glycol, using potassium hydroxide to vary the pH of the solution. The reaction takes place under high pressure—about 1800 pounds per square inch—and at more than 200° Celsius.
In the laboratory, the researchers use a heavy steel containment vessel that allowed them to analyze only the reaction results. To follow how the reaction took place, they needed to observe it in real time, which required development of a containment vessel small enough to allow for x-ray transmission while handling the high pressure and high temperature at the same time.
The result was a reaction vessel made of a high-strength quartz tube about a millimeter in diameter and about two inches long. After the cobalt hydroxide solution was added, the tube was spun to both facilitate the chemical reaction and average the x-ray signal. A small heater applied the necessary thermal energy and a thermocouple measured the temperature.
Ma and Chen, working with Wenqian Xu of Argonne National Laboratory and Lijun Wu of Brookhaven National Laboratory, used the setup for in situ synchrotron x-ray diffraction experiments during four separate trips to the X-ray Science Division 17-BM beamline of the Advanced Photon Source at Argonne, and the X14A x-ray beamline of the National Synchrotron Light Source (now the National Synchrotron Light Source II) at Brookhaven (both are Office of Science user facilities). X-rays passing through the reaction chamber to a two-dimensional detector provided continuous monitoring of the chemical reaction, which took about two hours to complete.
 “When they started forming a detectable spectrum we captured the x-ray diffraction spectrum and continued to observe it until the crystal cobalt formed,” Ma explained. “We were able to observe step-by-step what was happening from initial nucleation to the end of the reaction.”
“When they started forming a detectable spectrum we captured the x-ray diffraction spectrum and continued to observe it until the crystal cobalt formed,” Ma explained. “We were able to observe step-by-step what was happening from initial nucleation to the end of the reaction.”
Data obtained by varying the pH of the reaction produced a nanometric phase diagram showing where different combinations produced the two structures (Fig. 1).
The x-ray diffraction results confirmed the theoretical predictions and computational modeling done by Yifei Mo, an assistant professor in the A. James Clark School of Engineering at the University of Maryland. Mo and colleagues Adelaide Nolan and Shuo Zhang employed density functional theory to describe how the crystal would nucleate under differing conditions.
The success with cobalt suggests the methodology could be used to produce nanometric phase diagrams for other materials, including more complex alloys and oxides, Chen said.
“Our goal was to build a model and a systematic understanding about the formation of crystalline materials at the nanoscale,” he said. ‘Until now, researchers had been relying on empirical design to control growth of the materials. Now we can offer a theoretical model that would allow systematic prediction of what kinds of properties are possible under different conditions.”
As a next step, the Georgia Tech researchers plan to study alloys, to further improve the theoretical model and experimental approach.
The APS plays an important role for new approaches in material synthesis and is therefore expanding the capabilities for multi-modal approaches and complex sample environments using high-energy x-rays. After the upgrade of the APS, the program for structural science will enable new opportunities by combining small-angle and wide-angle scattering and working with very small beam sizes.
See: Xuetian Ma1, Adelaide M. Nolan2, Shuo Zhang2, Jianming Bai3, Wenqian Xu4, Lijun Wu3, Yifei Mo2*, and Hailong Chen1**, “Guiding Synthesis of Polymorphs of Materials Using Nanometric Phase Diagrams,” J. Am. Chem. Soc. 140, 17290 (2018). DOI: 10.1021/jacs.8b11029
Author affiliations: 1Georgia Institute of Technology, 2University of Maryland, 3Brookhaven National Laboratory, 4Argonne National Laboratory
Correspondence: *[email protected], **[email protected]
X.M. and H.C. thank the funding support from the U.S. National Science Foundation (NSF) under grant number 1605692 and 1739884, and the faculty start-up fund of the Georgia Institute of Technology. A.M.N., S.Z., and Y.M. acknowledge support from the NSF under award no. 1550423, and the computational facilities of the University of Maryland supercomputing resources, the Mary-land Advanced Research Computing Center (MARCC), and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by NSF award no. DMR150038. This research used resources of the National Synchrotron Light Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-AC02-98CH10886, and the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by National Laboratory under contract no. DE-AC02-06CH11357. TEM work was supported by the U.S. DOE Office Science-Basic Energy Sciences, Division of Materials Science and Engineering, under contract no. DE-SC0012704.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
