An intense research effort is under way worldwide on the replacing electrons with photons in solid-state switching devices. An international research team reported the outcome of experiments at the U.S. Department of Energy’s Advanced Photon Source (APS): the first-ever confirmed synthesis of an osmium sulfur dioxide ammine complex and the testing of its properties for solid-state optical switching. Such switches have applications in optical data storage and quantum computing, but their adoption has been hampered by interactivity problems between light and the material.
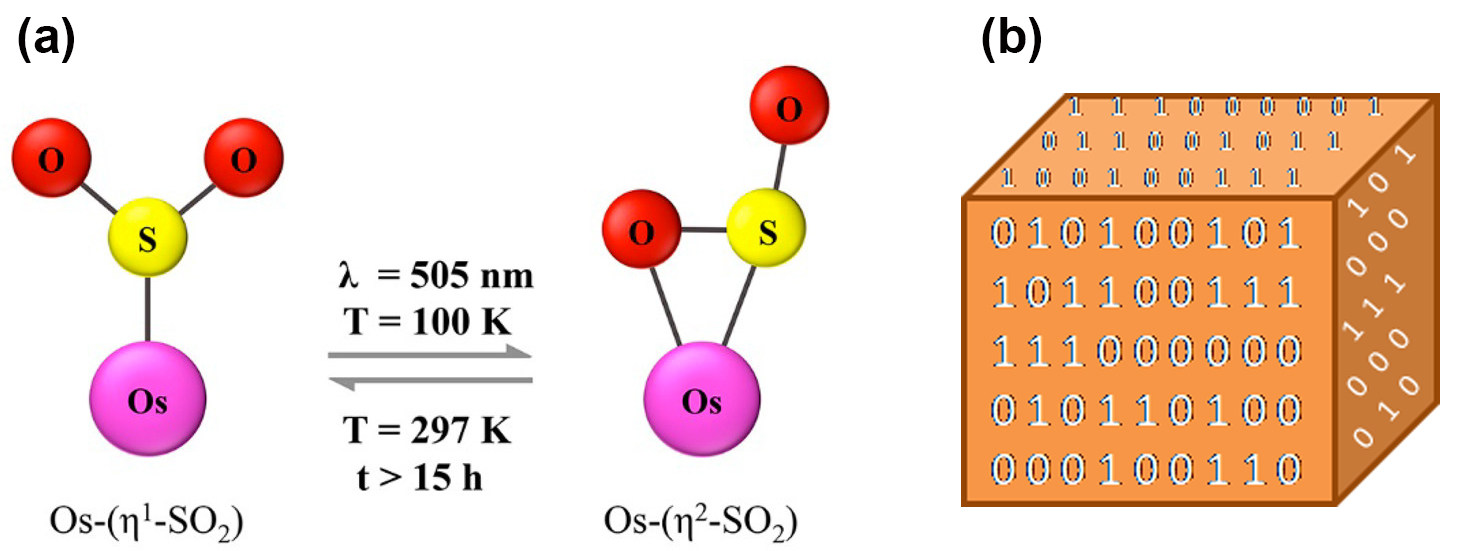
Single crystals of the [Os(NH3)5(SO2)]-[Os(NH3)5(HSO3)]Cl4, complex were prepared, collected by vacuum filtration, washed with CH3OH, and dried. By means of synchrotron x-ray diffraction at the ChemMatCARS beamline 15-ID-B,C,D of the Advanced Photon Source, the crystal structures of the dark- and light-induced states were then captured at a temperature of 100 Kelvin. (The APS is an Office of Science user facility at Argonne National Laboratory.) The results showed that stimulation of the crystal with 505-nm light for 2.5 hours induced a metastable state, whereby 9.3% of the SO2 ligands with one bond to the osmium (η1-SO2) was photo-converted to SO2 with two bonds (η2-SO2), as shown in Fig. 1a. The remaining 90.7% retained the dark-state structure. This photoisomerization caused little structural deformation to the crystal. These results suggest that osmium can better stabilize sulfur dioxide photoisomers than its ruthenium counterparts, which had been previously investigated.
The authors, from the University of Cambridge, the Rutherford Appleton Laboratory, Argonne, and The University of Chicago, further investigated the optical properties of the osmium sulfur dioxide ammine complex by means of concerted optical spectroscopy and microscopy, experiments that were performed at the Argonne Center for Nanoscale Materials, an Office of Science user facility located adjacent to the APS. The dark-state single crystal was found to undergo photoinduced “bleaching” upon 2.5 hours of exposure to 505-nm light at 100 Kelvin, where the optical absorption decreased by about 50% across most of the visible spectrum. The SO2 ligands appeared to be responsible for this bleaching effect. After warming to 275 Kelvin over 15 hours, the crystal exhibited progressive recovery of its optical absorption, but had not yet returned to the panchromatic absorption of the dark-state single crystal. By contrast, their previously investigated metastable ruthenium sulfur dioxide complex counterparts had returned to the dark state in a few seconds or minutes. These results demonstrate that the above osmium sulfur dioxide complex is a rare example of a material that can be developed into a promising osmium-based, solid-state, optical switch.
For example, one can imagine the dark-state SO2 ligand (η1-SO2) as a 0 and the photoisomer (η2-SO2) as a 1 in binary nomenclature. Since the molecular configurational changes in Fig. 1a take place within a single osmium-based crystal, one can then envision this crystal tailored with optically switchable 0s and 1s that express encoded forms of data – thus achieving three-dimensional optical data storage, as illustrated in Fig. 1b.
This capability is significant because bottom-row transition metals like osmium stand to offer linkage photoisomerism with the greatest photoconversion levels and thermal stability. Proposed future work is to produce chemical derivatives of the osmium sulfur dioxide complex that will generate even higher η2-SO2 photoconversion fractions and to search for other types of SO2 photoisomers in the osmium complex family. — Joseph E. Harmon
See: Jacqueline M. Cole1,2,3*, Jose de J. Velazquez-Garcia1, David J. Gosztola3, SuYin Grass Wang4, and Yu-Sheng Chen4, “η2‑SO2 Linkage Photoisomer of an Osmium Coordination Complex,” Inorg. Chem. 57, 2673 (2018). DOI: 10.1021/acs.inorgchem.7b03032
Author affiliations: 1University of Cambridge, 2STFC Rutherford Appleton Laboratory, 3Argonne National Laboratory, 4The University of Chicago
Correspondence: *[email protected].
J.M.C. thanks the 1851 Royal Commission of the Great Exhibition for the 2014 Fellowship in Design, hosted by Argonne National Laboratory, where work done was supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, and used research resources of the Center of Nanoscale Materials and the Advanced Photon Source (APS), Office of Science User Facilities operated for the DOE Office of Science by Argonne National Laboratory, supported by the U.S. DOE, all under contract No. DE-AC02-06CH11357. ChemMatCARS Sector 15 is principally supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), U.S. National Science Foundation (NSF), under grant number NSF/CHE-1346572. Use of the PILATUS3 X CdTe 1M detector is supported by the NSF under the grant number NSF/DMR-1531283. J.d.J.V. is indebted to the National Council of Science and Technology of Mexico (CONACyT) and the Cambridge Trust for a Ph.D. Scholarship (217553)
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
