The original Caltech press release by Kimm Fesenmaier can be read here
Not just anything is allowed to enter the nucleus, the heart of eukaryotic cells where, among other things, genetic information is stored. A double membrane, called the “nuclear envelope,” serves as a wall, protecting the contents of the nucleus. Any molecules trying to enter or exit the nucleus must do so via a cellular gatekeeper known as the nuclear pore complex (NPC), or pore, which exists within the envelope.
How can the NPC be such an effective gatekeeper — preventing much from entering the nucleus while helping to shuttle certain molecules across the nuclear envelope? Scientists have been trying to figure that out for decades, at least in part because the NPC is targeted by a number of diseases, including some aggressive forms of leukemia and nervous system disorders such as a hereditary form of Lou Gehrig's disease. Now a team of researchers from Caltech, The University of Chicago, and the Biochemistry Center of Heidelberg University (Germany), led by André Hoelz and working at three U.S. Department of Energy synchrotron light sources including the Advanced Photon Source (APS) at Argonne, has solved a crucial piece of the puzzle.
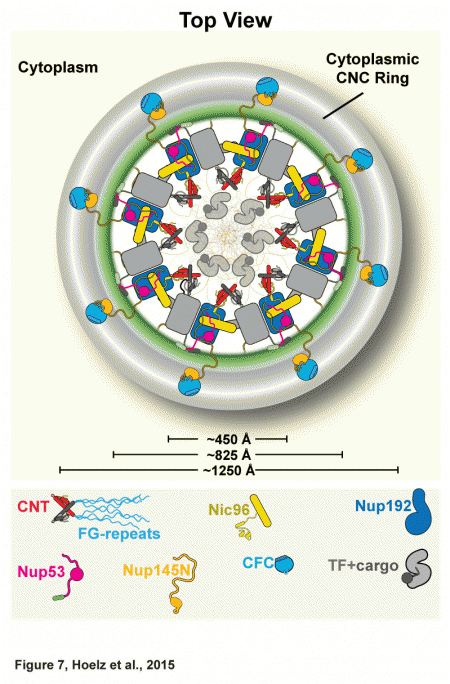
In February of this 2015, the team published a paper describing the atomic structure of the NPC's coat nucleoporin complex, a subcomplex that forms what they now call the outer rings (see the figure). Building on that work, the team has now solved the architecture of the pore's inner ring, a subcomplex that is central to the NPC's ability to serve as a barrier and transport facilitator. In order to determine that architecture, which determines how the ring's proteins interact with each other, the biochemists built up the complex in a test tube and then systematically dissected it to understand the individual interactions between components. Then they validated that this is actually how it works in vivo, in a species of fungus.
For more than a decade, other researchers have suggested that the inner ring is highly flexible and expands to allow large macromolecules to pass through. But now these researchers have shown that these models are incorrect and that these dilations simply do not occur.
Using an interdisciplinary approach, the team solved the architecture of this subcomplex and showed that it cannot change shape significantly. It is a relatively rigid scaffold that is incorporated into the pore and basically just sits as a decoration, like pom-poms on a bicycle. It cannot dilate.
Together, the inner and outer rings make up the symmetric core of the NPC, a structure that includes 21 different proteins. The symmetric core is so named because of its radial symmetry (the two remaining subcomplexes of the NPC are specific to either the side that faces the cell's cytoplasm or the side that faces the nucleus and are therefore not symmetric). Having previously solved the structure of the coat nucleoporin complex and located it in the outer rings, the researchers knew that the remaining components that are not membrane-anchored must make up the inner ring.
They started solving the architecture by focusing on the channel nucleoporin complex, or channel, which lines the central transport channel and is made up of three proteins, accounting for about half of the inner ring. This complex produces filamentous structures that serve as docking sites for specific proteins that ferry molecules across the nuclear envelope.
The biochemists employed bacteria to make the proteins associated with the inner ring in a test tube and mixed various combinations until they built the entire subcomplex. Once they had reconstituted the inner ring subcomplex, they were able to modify it to investigate how it is held together and which of its components are critical, and to determine how the channel is attached to the rest of the pore.
The research team found that the channel is attached at only one site. This means that it cannot stretch significantly because such shape changes require multiple attachment points. An electron microscopy study of the NPC published in 2013 by Martin Beck's group at the European Molecular Biology Laboratory in Heidelberg, Germany, indicated that the central channel is bigger than previously thought and wide enough to accommodate even the largest cargoes known to pass through the pore.
When the researchers introduced mutations that effectively eliminated the channel's single attachment, the complex could no longer be incorporated into the inner ring. After proving this in the test tube, they also showed this to be true in living cells.
The entire complex is a very complicated machine to assemble. Nature has found an elegant way to wait until the very end of the assembly of the nuclear pore to incorporate the channel. By incorporating the channel, a barrier is immediately formed, and regulated transport can occur through the pore. Prior to the channel's incorporation, there is simply a hole through which macromolecules can freely pass.
Next, the team used x-ray crystallography at the 23-ID-D beamline of the National Institute of General Medical Sciences and National Cancer Institute (GM/CA-XSD) facility at the APS, the Stanford Synchrotron Radiation Laboratory (SSRL) beamline 12-2, and the Advanced Light Source (ALS) beamline 8.2.1 to determine the structure of the channel nucleoporin subcomplex bound to the adaptor nucleoporin Nic96, which is its only nuclear pore attachment site. (The APS, SSRL, and ALS are all Office of Science user facilities.) Because the NPC is a large and complex molecular machine that also has many moving parts, the researchers used an engineered antibody to essentially “superglue” many copies of the complex into place to form a nicely ordered crystalline sample. Then they analyzed hundreds of samples using Caltech's Molecular Observatory. Eventually, they were able to determine the size, shape, and position of all the atoms of the channel nucleoporin subcomplex and its location within the full NPC.
The researchers also solved a number of crystal structures from other parts of the NPC and determined how they interact with components of the inner ring. In doing so they demonstrated that one such interaction is critical for positioning the channel in the center of the inner ring. They found that exact positioning is needed for the proper export from the nucleus of mRNA and components of ribosomes, the cell's protein-making complexes, rendering it critical in the flow of genetic information from DNA to mRNA to protein.
Now that the architectures of the inner and outer rings of the NPC are known, getting an atomic structure of the entire symmetric core is “a sprint to the summit.”
A video that features a rotating three-dimensional crystal structure of the fungal channel nucleoporin complex bound to the adaptor nucleoporin Nic96 can be seen here. This interaction is the complex's sole site of attachment to the rest of the inner ring of the NPC. The channel nucleoporin complex borders the central transport channel and fills it with filamentous structures (phenylalanine-glycine repeats) that form a diffusion barrier and provide docking sites for proteins that ferry molecules across the nuclear envelope. Credit: Andre Hoelz/Caltech and Science
See: Tobias Stuwe1, Christopher J. Bley1, Karsten Thierbach1, Stefan Petrovic1, Sandra Schilbach1, Daniel J. Mayo1, Thibaud Perriches1, Emily J. Rundlet1, Young E. Jeon1, Leslie N. Collins1, Ferdinand M. Huber1, Daniel H. Lin1, Marcin Paduch2, Akiko Koide2, Vincent Lu2, Jessica Fischer3, Ed Hurt3, Shohei Koide2, Anthony A. Kossiakoff2, and André Hoelz1* , “Architecture of the fungal nuclear pore inner ring complex,” published online in Sciencexpress, 27 August 2015. DOI: 10.1126/science.aac9176
Author affiliations: 1California Institute of Technology, 2University of Chicago, 3Biochemistry Center of Heidelberg University
Correspondence: *[email protected]
T.S. was supported by a Postdoctoral Fellowship of the Deutsche Forschungsgemeinschaft. S.P. and D.H.L are Amgen Graduate Fellows, supported through the Caltech-Amgen Research Collaboration. F.M.H. was supported by a Ph.D. student fellowship of the Boehringer Ingelheim Fonds. S.K. was supported by National Institutes of Health (NIH) Awards R01-GM090324 and U54-GM087519 and by the University of Chicago Comprehensive Cancer Center (P30-CA014599). A.A.K. was supported by NIH awards U01-GM094588 and U54-GM087519 and by Searle Funds at The Chicago Community Trust. A.H. was supported by Caltech startup funds, the Albert Wyrick V Scholar Award of the V Foundation for Cancer Research, the 54th Mallinckrodt Scholar Award of the Edward Mallinckrodt Jr. Foundation, a Kimmel Scholar Award of the Sidney Kimmel Foundation for Cancer Research, a Camille-Dreyfus Teacher Scholar Award of The Camille & Henry Dreyfus Foundation, and NIH grant R01-GM111461. GM/CA-XSD has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.