Lithium-ion (Li-ion) batteries provide the juice for many portable electronic devices. These rechargeable batteries offer several advantages, such as a high energy density and low maintenance. But like all batteries, they degrade over time. The constant charging and discharging of a lithium-ion battery causes volume contraction and expansion inside electrode components. This mechanical strain can eventually lead to cracking and is one of the main causes of Li-ion battery failure. To better understand the impact of strain, researchers have produced the first three-dimensional mapping of atom-level strain inside metal oxide nanoparticles that store and release charge. The experiments, performed at the U.S. Department of Energy’s Advanced Photon Source, show that strain is not uniform across these battery components. These precise nanoscale images may help in optimizing the shape and size of nanoparticles, so that strain is less of a drain on battery life.
Like most batteries, the Li-ion variety consist of a positive cathode and a negative anode separated by an electrolyte. The cathode in this case is made of a lithium metal oxide that allows lithium ions – which are the battery’s charge carriers – to leave and reenter. To facilitate this ion movement, the metal oxide is routinely fabricated as a powder of small particles that are micrometers or nanometers in size. During charging, lithium ions diffuse out of these particles and travel through the electrolyte to the anode, which is often some form of carbon such as graphite. The loss of lithium in the cathode particles results in a contraction of their crystal structure. When the battery starts to discharge, the lithium ions flow back into the particles, causing them to re-expand.The strain from this cycling of ions into and out of the cathode material may induce structural damage. For this reason, battery manufacturers are interested in understanding how and where strain occurs.
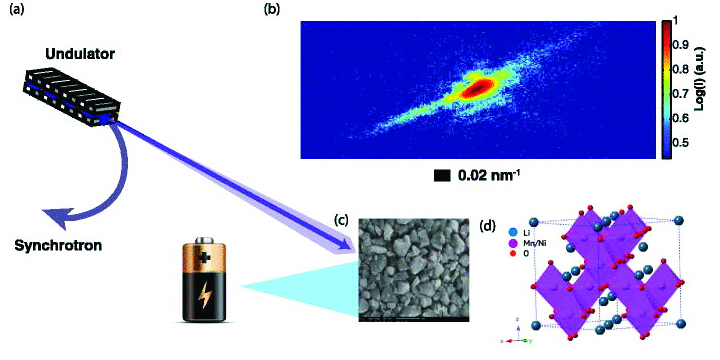
To see the strain inside nanoscale particles, researchers from the University of California, San Diego; Argonne National Laboratory, Los Alamos National Laboratory, and New Mexico State University employed coherent x-ray diffraction imaging (CXDI) at the X-ray Science Division 34-ID-C beamline at the Argonne APS, an Office of Science user facility. This relatively new technique was developed to take images of nano-sized objects, like gold nanoparticles and semiconductor quantum dots. The beam used in CXDI is formed by extracting a small fraction of coherent light from the APS x-ray output (see top of the figure). The 34-ID-C beamline is optimized for performing CXDI, with a set of curved Kirkpatrick-Baez mirrors for focusing the synchrotron light on a sample. When the coherent x-rays scatter off the sample’s atoms, they produce a high-precision diffraction pattern, which can be transformed – through a computer algorithm – into a three-dimensional map of the crystal structure in the sample.
For their experiments, the research team chose to look at cathode materials called spinels, which are used in special high-performance batteries. Spinels are a type of oxide with a specific cubic crystal lattice structure. In spinels containing lithium, this cubic structure allows lithium ions to diffuse out in three different directions, as compared to a single direction in other metal oxide materials. This greater ion mobility results in a higher voltage for spinel-based batteries than for the more common lithium cobalt oxide battery. A further advantage of spinels is that they don’t require environmentally hazardous materials in their fabrication.
As described in Applied Physics Letters, the researchers performed CXDI experiments on nanoparticles made from the spinel material lithium-nickel-manganese-oxide. By examining the asymmetrical shape of Bragg peaks in the diffraction pattern, the team recovered the strain inside single nanoparticles that were isolated from both a prepared powder sample and a coin cell battery (see bottom of the figure).
The maximum strain was measured to be around one part in 103, which is about ten times the strain found in gold nanoparticles of similar size. Interestingly, the strain was not uniform across the nanoparticles. The research team assumed this inhomogeneity was due to differing concentrations of lithium ions in the crystal structure. This would imply that lithium diffuses out of the nanoparticles in a non-uniform way, contradicting certain theoretical models that predict diffusion proceeds uniformly from the surface to the core.
Subsequent work with CXDI techniques has shown how strain evolves over time in these spinel materials as they cycle through charge and discharge. By experimenting with different nanoparticle shapes and sizes, the researchers hope to find ways to limit the effects of strain. — Michael Schirber
See: A. Ulvestad1, H.M. Cho1, R. Harder2, J.W. Kim1, S.H. Dietze,1 E. Fohtung3,4, Y.S. Meng1, and O.G. Shpyrko1, “Nanoscale strain mapping in battery nanostructures,” App. Phys. Lett. 104, 073108 (2014). DOI: /10.1063/1.4866030
Author affiliations: 1University of California, San Diego; 2Argonne National Laboratory; 3Los Alamos National Laboratory; 4New Mexico State University
Correspondence: * [email protected]
This work was supported by the U.S. Department of Energy, Office of Science-Basic Energy Sciences, under Contract No. DE-SC0001805 and by the UCSD Chancellor’s Interdisciplinary Award. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.