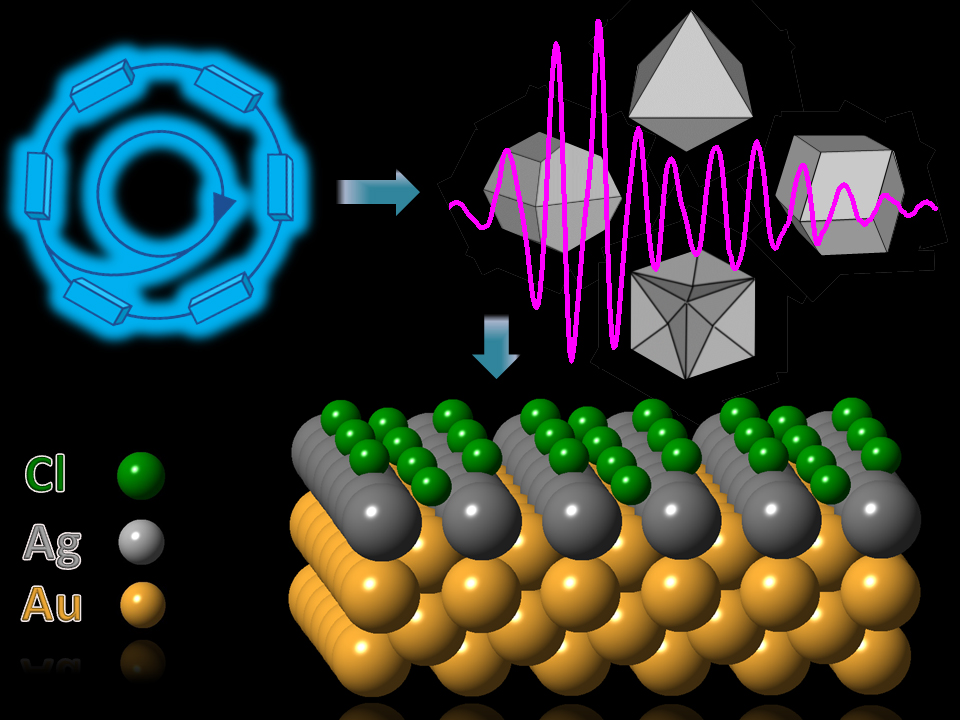
Nanocrystals of metals such as gold can be used to make more-sensitive sensors and other devices for biomedical applications. The electronic and optical properties of such crystals—which wavelength of light they respond to, for instance—can be tuned by changing the shape of the nanocrystals’ facets. Therefore, understanding the mechanisms that control those shapes will be important for those applications. Now a team of researchers has used the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility, to show how a mixture of silver and chloride can determine the facet shapes of gold nanoparticles, providing the recipe for controlling the properties of the crystals.
The researchers from Dalhousie University (Canada); Northwestern University; and the University of California, Riverside created nanocrystals approximately 50 to 100 nm in diameter by taking gold seed crystals and putting them in a mixture containing cetyltrimethylammonium chloride, hydrochloric acid, ascorbic acid, hydrogen tetrachloroaurate, and varying amounts of silver nitrate, between 1 and 10 micromoles. The concentration of silver nitrate controlled the final shape of the crystal, and the different combinations produced prisms, 8- and 12-sided crystals, and cubes with concave surfaces.
To study the relationship between the concentration and the shape, the researchers subjected the crystals to x-ray absorption spectroscopy at the X-ray Science Division 20-BM-B x-ray beamline at the Argonne APS. This beamline produces an intense x-ray beam at selected energy levels. They also employed the SXRMB beamline at the Canadian Light Source. They performed extended x-ray absorption fine structure experiments, which revealed the atomic structure of the crystalline surface and showed the bonding mechanisms between silver and chlorine, silver and gold, and gold and gold atoms; and x-ray absorption near-edge structure experiments to learn about the oxidation state of the silver.
The studies not only confirmed that the concentration of silver determines the shape of the nanocrystals, but also provides clues as to the role played by the chloride. The chloride helps stabilize the silver coating formed on gold nanoparticles by binding to the surface. It turned out that the silver-chloride combinations in each of the four different nanocrystals studied had different bonding behavior, allowing stabilization of the silver on different types of facets. It was already known that effect could not be achieved using other halides, such as bromide or fluoride, and this work showed that it is the flexibility of the chloride’s bonding mechanisms that allow it to happen here.
Researchers also looked at the role of the chemical procedure in the crystal growth. On three of the four types of crystals, the team used a unique preparation method, underpotential deposition (UPD), in which the reduction potential (voltage) of silver was just at the edge of being strong enough to start the reaction, slowing the growth. The x-ray studies showed that in those UPD-produced crystal types, the silver formed a single-layer coating on top of the gold, but in the type with the faster non-UPD growth, silver atoms penetrated the surface of the gold and formed an alloy, affecting the nanocrystal’s properties. The role of the chloride had not been shown before because, being such a light molecule, it could not be easily studied with other techniques, such as electron microscopy.
To confirm their findings, the researchers ran computer simulations of the structures using density functional theory modelling. Not only did the models match the experimental x-ray data, they also helped to plot the actual coverage of chloride on the surface of the nanocrystals.
The researchers hope to do similar studies on nanocrystals made with other metals. It could be useful, for example, to study catalysts such as platinum or palladium, where the shape of the crystal affects the rate of catalysis. — Neil Savage
See: J. Daniel Padmos1, Michelle L. Personick2, Qing Tang3, Paul N. Duchesne1, De-en Jiang3, Chad A. Mirkin2, and Peng Zhang1*, “The surface structure of silver-coated gold nanocrystals and its influence on shape control,” Nat. Commun. 6, 7664-1 (2015). DOI: 10.1038/ncomms8664
Author affiliations: 1Dalhousie University, 2Northwestern University, 3University of California, Riverside
Correspondence: *[email protected]
The financial support of this work from the Natural Sciences and Engineering Research Council (NSERC) Canada is kindly acknowledged by J.D.P. and P.Z. The synthetic portion of the work was supported by the National Science Foundation’s (NSF’s) MRSEC program (DMR-1121262) at the Materials Research Center of Northwestern University. In addition, M.L.P. gratefully acknowledges support from the NSF through a Graduate Research Fellowship and from the U.S. Department of Defense through the National Defense Science & Engineering Graduate Fellowship Program (32 CFR 168a). The computational portion of this work was supported by the University of California, Riverside and used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy (DOE) under Contract No. DE-AC02-05CH11231. The Canadian Light Source (CLS) is supported by NSERC, the Canadian Institutes of Health Research, and the University of Saskatchewan. APS Sector 20, which is managed by XSD in partnership with the CLS, is funded by the U.S. DOE Office of Science, and by the Natural Sciences and Engineering Research Council of Canada and the University of Washington via the CLS. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.