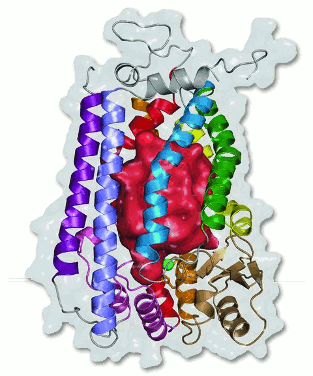
Researchers have determined the molecular structure of a protein whose mutations have been linked to several early aging diseases, and side effects for common human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) medications. This breakthrough could eventually help researchers develop new treatments for these early-aging diseases and redesign AIDS medications to avoid side effects such as diabetes. The research was carried out at the Southeastern Regional Collaborative Access Team (SER-CAT) facility at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory.
The researchers from the University of Virginia School of Medicine, the Hauptman-Woodward Medical Research Institute, and the University of Rochester School of Medicine and Dentistry determined the molecular structure of the enzyme Ste24p. Their Membrane Protein Structural Biology Consortium is funded by the National Institutes of Health Protein Structure Initiative, which supports the determination of molecular structures of biomedically important target proteins. Their findings were published March 29 in the journal Science.
The nucleus of a cell is lined with a mesh-like structure called lamin that serves as a scaffold for maintaining nuclear shape and function. Lamin requires the action of another protein, called Ste24p protease, which removes specific parts of the lamin filament and enables proper assembly of the mesh. The research team determined the structure of a Ste24p protein found in a close relative of baker’s yeast, whose properties are interchangeable with the same protein found in humans.
These experiments required to solve the structure of Ste24p were performed remotely at the Southeastern Regional Collaborative Access Team x-ray beamline 22-ID at the APS. Working from Charlottesville, UVA researchers can log onto a computer program to remotely control the robots sample-handlers at the SER-CAT facility to perform research and to collect data.
In humans, improper processing and assembly of lamin lead to a group of diseases known as laminopathies, which include muscular dystrophy, cardiomyopathies, and a collection of rare accelerated aging diseases in children called progerias. The research team focused on Ste24p because defects in this protein in humans are responsible for a number of progerias.
In addition, some frequently used HIV and AIDS medications utilized in highly active antiretroviral therapy (HAART) also interact with Ste24p, which may lead to alterations in the way patients taking the drugs metabolize fat and may lead to treatment side effects that can include insulin resistance and diabetes, Pryor said.
Knowing how the protein is structured could aid researchers in developing drugs to treat progerias and could assist in the redesign of HAART medications to avoid the treatment side effects.
Ste24p is a biomarker for normal aging in humans – as humans get older, this protein works less effectively. So this mapping could provide insight for researchers into the human aging process.
See: Edward E. Pryor, Jr.1; Peter S. Horanyi1; Kathleen M. Clark1,2; Nadia Fedoriw1,2; Sara M. Connelly1,2; Mary Koszelak-Rosenblum1,3; Guangyu Zhu1,3; Michael G. Malkowski1,3,4; Michael C. Wiener1*; and Mark E. Dumont1,2,5**; “Structure of the Integral Membrane Protein CAAX Protease Ste24p,” Science 339, 1600 (29 March 2013). DOI: 10.1126/science.1232048
Author affiliations: 1University of Virginia, Charlottesville, 2University of Rochester School of Medicine and Dentistry, 3Hauptman-Woodward Institute, 4State University of New York at Buffalo, 5University of Rochester School of Medicine and Dentistry
Correspondence: *[email protected], *[email protected]
This work was supported by Protein Structure Initiative: Biology grant U54 GM094611 (National Institute of Health) for membrane protein structural genomics. SER-CAT supporting institutions may be found at www.ser-cat.org/members.html. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under Contract No. DE-AC02-06CH11357.
The original University of Virginia Health System news release by Josh Barney can be read here.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.