The original Emory University Woodruff Health Sciences Center press release by Quinn Eastman can be read here.
When doctors hurl toxic death at cancer cells, often a few will survive and come back. A family of enzymes called KDM5 histone demethylases is emerging as important for this resilience, and drugs that inhibit KDM5 enzymes could be active in treating several types of cancer. A team of investigators from Emory University, the Yale School of Medicine, and the and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health used the U.S. Department of Energy’s Advanced Photon Source (APS) to obtain detailed structural information, showing how inhibitors of the KDM5 family interact with their targets.
Their findings, published in Cell Chemical Biology, could inform efforts to design more potent and selective anticancer drugs.
Drugs are sometimes compared to keys, because they need to fit precisely into the enzymes they target. But there is some wiggle room, since usually more than one drug can inhibit a given enzyme. In addition, there are several families of histone demethylase enzymes, and an ongoing challenge is developing drugs that specifically inhibit KDM5 enzymes.
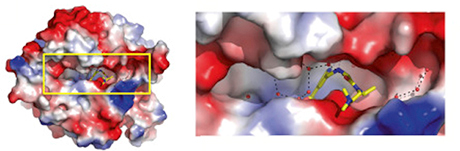
Using x-ray crystallography at the Southeast Regional Collaborative Access Team 22-ID-D x-ray beamline at the APS, an Office of Science user facility at Argonne, the scientists led by biochemists John Horton and Xiaodong Cheng, at Emory University School of Medicine and Winship Cancer Institute were able to see how eight different inhibitory compounds squeeze into the active site of the one member of the KDM5 family, KDM5A. In doing so, they could also see how additional potential drugs, not yet synthesized, might fit the contours of the enzyme more closely.
Scientists at NCATS identified and synthesized most of the inhibitors, several of which are being developed and tested by pharmaceutical companies. Qin Yan’s laboratory at Yale examined the growth-stopping effects of one KDM5 inhibitor on breast cancer cells.
Histones are spool-like proteins that DNA wraps around in the cell nucleus. For a particular gene to be active and transcribed into RNA, histones have to be moved out of the way. A variety of enzymes prepare for the parade (RNA transcription) by adding signposts: chemically tweaking the histones.
KDM5 demethylases remove modifications (a –CH3 methyl group on lysine 4 of histone H3) that are a sign of a gene being active. Thus, they help maintain genes in an “off” state. KDM5 inhibitors thus are likely to keep some genes on – but they won’t necessarily act the same in all cell types.
In this paper, the investigators focused on the effects on breast cancer cells, while other laboratories have shown that KDM5 enzymes are linked to drug tolerance or resistance in lung cancer, glioblastoma and melanoma.
Qin Yan’s team at Yale found that only some types of breast cancer cells’ growth is stopped by KDM5 inhibitors. For example, a cell line that was HER2+ (a marker that indicates likely response to the drug trastuzumab) is, but another cell line, representative of a “triple-negative” is not.
“We had a clue already that some breast cancer cell lines are more sensitive to KDM5 gene knockdown. This may indicate that KDM5 inhibitors will be more active toward HER2+ and luminal-type cells and less toward basal cells,” said Yan.
See: John R. Horton1, Xu Liu1, Molly Gale2, Lizhen Wu2, John R. Shanks1, Xing Zhang1, Philip J. Webber1, Joshua S.K. Bell1, Stephen C. Kales3, Bryan T. Mott3, Ganesha Rai3, Daniel J. Jansen3, Mark J. Henderson3, Daniel J. Urban3, Matthew D. Hall3, Anton Simeonov3, David J. Maloney3, Margaret A. Johns1, Haian Fu1, Ajit Jadhav3, Paula M. Vertino1, Qin Yan2*, and Xiaodong Cheng1**, “Structural Basis for KDM5A Histone Lysine Demethylase Inhibition by Diverse Compounds,” Cell Chem. Biol. 23, 1 (July 21, 2016). DOI: 10.1016/j.chembiol.2016.06.006
Author affiliations: 1Emory University, 2Yale School of Medicine, 3National Institutes of Health
Correspondence: *[email protected], **[email protected]
This project has been funded in part with Federal funds from the National Cancer Institute (NCI), NIH, under NCI Chemical Biology Consortium Contract No. HHSN261200800001E (to H.F.), NIH grants (GM114306-02 to X.C. and CA077337 to P.M.V.), American Cancer Society Research Scholar Grant (RSG-13-384-01-DMC to Q.Y.) and DoD Breast Cancer Research Program Award (W81XWH-14-1-0308 to Q.Y.), National Science Foundation Graduate Research Fellowship (DGE-1122492 to M.G.), Leslie H. Warner Postdoctoral Fellowship (to L.W.), NIH predoctoral NRSA F31 fellowship (CA186676 to J.S.K.B.), developmental funds (to X.C. and P.M.V.) from the Winship Cancer Institute of Emory University Cancer Center Support Grant P30-CA138292, and funds from the Arthur and Sarah Merrill Foundation (to X.C.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The Department of Biochemistry of Emory University School of Medicine supported the use of the Southeast Regional Collaborative Access Team synchrotron beamlines at the Advanced Photon Source of Argonne National Laboratory. X.C. is a Georgia Research Alliance Eminent Scholar. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.