This article is based upon the Johns Hopkins University School of Medicine press release, which can be read here.
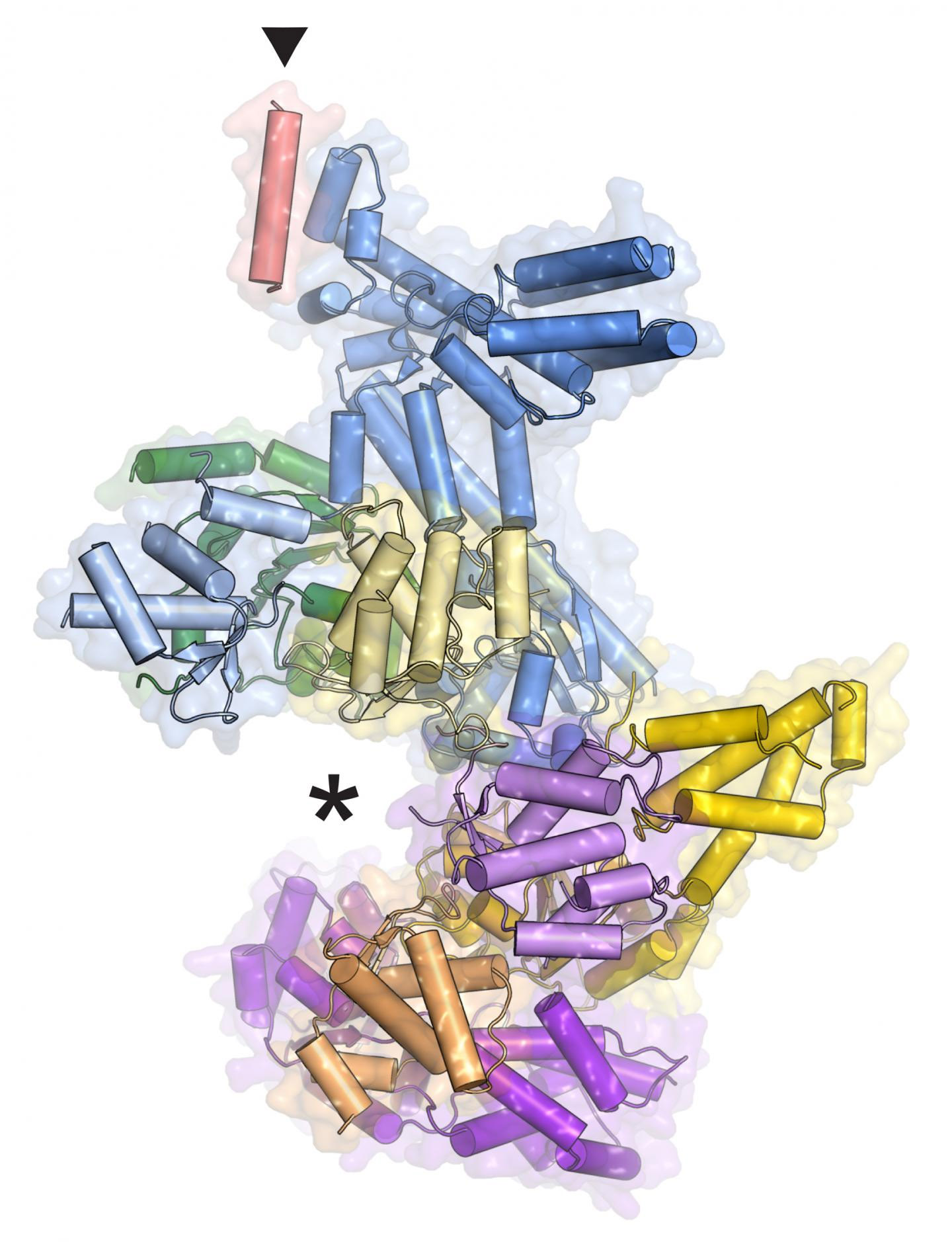
An intricate three-dimensional model of the complex protein that helps prepare DNA for duplication is the result of research at three U.S. Department of Energy x-ray light sources, including the Advanced Photon Source at Argonne National Laboratory.The model of this protein machine, called ORC, has helped build a “profile” of the activities of this crucial protein of interest. But the new information has uncovered another mystery: ORC's structure reveals that it is not always “on” as was previously thought, and no one knows how it turns on and off.
“Even though the ORC protein machinery is crucial to life, we didn't know much about how it works,” said James Berger, professor of biophysics and biophysical chemistry at the Johns Hopkins School of Medicine and a co-author, with Franziska Bleichert of Johns Hopkins, and Michael R. Botchan of the University of California, Berkeley, of the study that was published in Nature. “By learning what [ORC] looks like, down to the arrangement of each atom, we can get a sense of where it interacts with DNA and how it does its job,’ said Berger.
Multicelled organisms grow when their cells divide into two. However, before a cell can divide, it has to make copies of its parts for the new cell. Since DNA's information is sealed inside its double strands, a specialized machine, called the replisome, must unseal the strands before the information can be accessed and copied.
A key piece of the replisome is a motor, termed MCM, which unwinds paired DNA strands. MCM is a closed protein ring that must be opened up before it can encircle the long strands of DNA. ORC, the origin recognition complex, solves that problem. It cracks open the MCM's circle so that it can fit around the DNA and unwind it.
It was previously known that ORC is a six-piece protein complex, with five of the pieces forming a slightly opened ring and the sixth, Orc6, forming a tail. Mistakes in Orc6 cause assembly problems, which affect the function of the whole machine and contribute to a dwarfism disorder called Meier-Gorlin syndrome. To learn more about how the complex works, co-author Franziska Bleichert, a postdoctoral fellow in Berger's laboratory at Johns Hopkins, extracted the protein from fruit fly cells and immobilized it by coaxing it into tiny crystals. She then analyzed the crystals’ structures utilizing high-energy x-ray beams from the National Institute of General Medical Sciences and National Cancer Institute (GM/CA-XSD) beamline 23-ID-B at the Advanced Photon Source, beamline 8.3.1 at the Advanced Light Source at Lawrence Berkeley National Laboratory, and beamline X25 at the National Synchrotron Light Source at Brookhaven National Laboratory. All three laboratories are Office of Science user facilities. The resulting data allowed her to reconstruct the precise shape of the proteins, atom by atom, on the scale of billionths of an inch.
Her model reveals exactly where Orc6 connects to the ring of ORC and explains how mistakes in that protein wreak havoc, though why dysfunctional ORC should cause dwarfism is still a mystery.
The three-dimensional model also showed the existence of an unexpected regulatory mechanism. It was previously thought that ORC was always on, just not always present in the nucleus where it does its work. The model shows that it can exist in an inactive state, raising the question: How does it turn on and off?
“In hindsight, it's not surprising that there is another level of regulation for ORC,” said Berger. For example, “As soon as an egg cell is fertilized, it has to jump into action to create the embryo through multiple rounds of cell division, which first requires DNA replication. This inactive state might allow egg cells to stockpile ORC inside the nucleus so it's available when needed.” His team plans to test this idea soon.
See: Franziska Bleichert1, Michael R. Botchan2**, and James M. Berger1*, “Crystal structure of the eukaryotic origin recognition complex,” Nature, published online 11 March 2015. DOI: 10.1038/nature14239
Author affiliations: 1Johns Hopkins School of Medicine, 2University of California Berkeley
Correspondence: * [email protected], ** [email protected]
This work was supported by the National Institutes of Health (GM071747 to J.M.B. and CA R37-30490 to M.R.B.) and by a fellowship from the UC Berkeley Miller Institute for Basic Research in Science (to F.B.). GM/CA-XSD has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.