The human immunodeficiency virus type 1 (HIV-1) was responsible for 1.5 million deaths around the world in 2013. Although researchers have been able to develop anti-retroviral therapies that allow patients to live longer with the disease, it has been a struggle to develop an effective vaccine that prevents the spread of infection. Theoretically, the problem is one we think we know how to solve. HIV-1 has a single spike protein on its surface called Env that mediates viral fusion and entry into host cells. If we can prime the immune system to recognize Env and neutralize it, we should be able to stop HIV-1 in its tracks. However, up to this point, HIV-1 has used molecular trickery to evade our efforts to bolster anti-HIV-1 immunity. Now, new structural data collected at the U.S. Department of Energy's Advanced Photon Source (APS) by a research team from universities in the United States and South Africa, has revealed the basis for some of HIV-1’s elusiveness. Their work maps sites of potential vulnerability identified in the sera of patients who have made neutralizing antibodies to HIV-1, opening the door to the design of potentially more effective vaccines against HIV.
One of the challenges in our understanding of HIV-1 has been in obtaining good structural information on the Env protein. This protein is a complex fusion machine composed of three copies of a subunit called gp120 (receptor binding domain) and three copies of a subunit called gp41 (fusion domain). These assemble on the surface of HIV-1 in a closed pre-fusion mature state that is activated upon receptor binding to undergo a dramatic conformational change in which the gp41 Env protein pierces the host cell and merges the viral membrane with the host cell membrane. This process allows the HIV-1 genome entry to the host cell cytoplasm.
Researchers believe that neutralizing antibodies that target the pre-fusion state of Env have the best chance of conferring lasting immunity. However, because the pre-fusion association between gp120 and gp41 is fragile and because the post-fusion state is more energetically favored, it has been difficult to crystallize the pre-fusion state. The researchers in this study, from the National Institutes of Health, the National Institute for Communicable Diseases of the National Health Laboratory Service (South Africa), the University of North Carolina at Chapel Hill, the Duke University School of Medicine and the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery at Duke University, the University of the Witwatersrand (South Africa), the University of KwaZulu-Natal (South Africa), the Yale University School of Medicine, and the Weill Cornell Medical College of Cornell University used their own molecular trick to overcome this They were able to crystallize the gp120/gp41 complex by stabilizing it with two antibodies that recognize different parts of the structure.
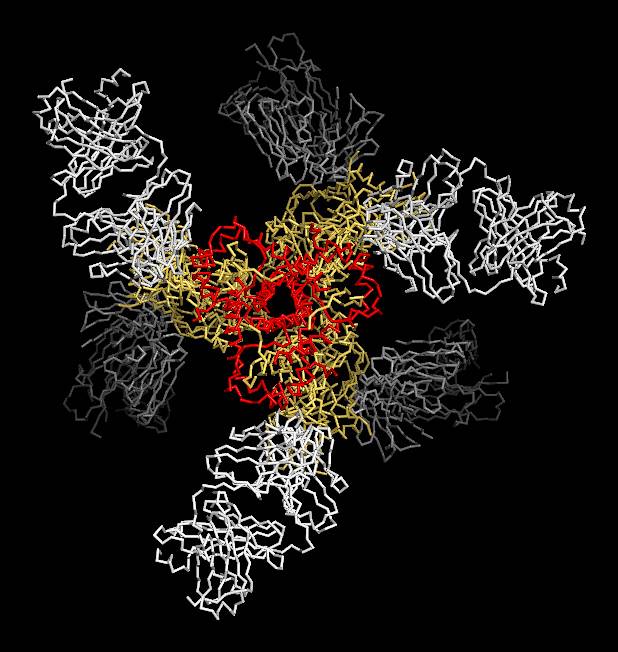
The structural data obtained by utilizing the Southeast Regional Collaborative Access Team 22-ID-D x-ray beamline at the Argonne National Laboratory APS, an Office of Science user facility, show a number of interesting features of the complex. The subunits are generally oriented into a propeller-like shape, and each molecule of gp41 in the structure forms a kind of collar through which the ends of gp120 extend towards the viral membrane (see the figure). The collar is formed by helices and loops of gp41 and is fastened with a molecular clasp.
Comparison to the structure of the post-fusion complex shows that gp120 binding to the host cell causes the gp41 collar to rearrange and become a pair of extended helices that force a molecular spike through the host membrane and pull the two membranes together.
The team compared the HIV-1 fusion machine to other known viral fusion machines and found that the topology of the interaction is similar to the influenza, respiratory syncytial, and Ebola viruses. But what makes it so hard to recognize immunologically?
The answer lies, in part, in sugar modifications (glycosylations) that are added to the surface of the molecule after it is made. Modeling showed that glycosylation, which is less likely to cause an immune response, leaves only 3% of the protein surface free for antibodies to bind. Compared to influenza at 14% and respiratory syncytial virus at 48%, that is precious little space for mounting an immune response. In addition, the exposed surface of HIV-1 exhibits extreme genetic variability that makes it even more difficult to target effectively with antibodies.
The final part of this study was to look at some immunological successes. Despite HIV-1’s molecular trickery, some patients have developed neutralizing antibodies to HIV-1. The team looked at these to see where they bind and found that, in general, successful antibodies can accommodate HIV-1’s variability in their recognition pattern and incorporate recognition of glycosylation as well.
The researchers hope that this new information will provide the edge they need to finally block HIV-1’s spike.
— Sandy Field
See: Marie Pancera1, Tongqing Zhou1, Aliaksandr Druz1, Ivelin S. Georgiev1, Cinque Soto1, Jason Gorman1, Jinghe Huang1, Priyamvada Acharya1, Gwo-Yu Chuang1, Gilad Ofek1, Guillaume B.E. Stewart-Jones1, Jonathan Stuckey1, Robert T. Bailer1, M. Gordon Joyce1, Mark K. Louder1, Nancy Tumba2, Yongping Yang1, Baoshan Zhang1, Myron S. Cohen3, Barton F. Haynes4, John R. Mascola1, Lynn Morris2,5,6, James B. Munro7, Scott C. Blanchard8, Walther Mothes7, Mark Connors1, and Peter D. Kwong1*, “Structure and immune recognition of trimeric pre-fusion HIV-1 Env,” Nature 514, 455 (23 October 2014). DOI: 10.1038/nature13808
Author affiliations: 1National Institutes of Health, 2National Institute for Communicable Diseases of the National Health Laboratory Service, 3University of North Carolina at Chapel Hill, 4Duke University School of Medicine and the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery at Duke University, 5University of the Witwatersrand, 6University of KwaZulu-Natal, 7Yale University School of Medicine, 8Weill Cornell Medical College of Cornell University
Correspondence: * [email protected]
Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); and by grants from the Division of AIDS, NIAID, NIH (R21-AI100696, CHAVI-AI0678501, and CHAVI-Immunogen Discovery-AI100645), from the National Institutes of General Medical Sciences (PO1-GM56550 and RO1-GM098859), from the Irvington Fellows Program of the Cancer Research Program, and from the International AIDS Vaccine Initiative’s (IAVI’s) Neutralizing Antibody Consortium. IAVI’s work is made possible by support from many donors including: the Bill & Melinda Gates Foundation; the Ministry of Foreign Affairs of Denmark; Irish Aid; the Ministry of Finance of Japan; the Ministry of Foreign Affairs of the Netherlands; the Norwegian Agency for Development Cooperation; the UK Department for International Development; and the United States Agency for International Development. The full list of IAVI donors is available at http://www.iavi.org. The Southeast Regional Collaborative Access Team supporting institutions can be found at www.ser-cat.org/members.html. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.