Insects and their relatives are arguably the most colorful group of animals on the planet. The brilliant green of a butterfly wing or the shimmering gold from a beetle shell are often due to arrays of nanoscale structures that scatter light only at specific wavelengths and at certain angles. A new study has cataloged the vast variety of structural color mechanisms in insects, as well as spiders. Using the U.S. Department of Energy’s Advanced Photon Source (APS), the researchers show that the so-called “photonic nanostructures” in these organisms have shapes similar to self-organizing membrane structures found inside cells, as well as in detergents, and certain polymers. But unlike artificial polymeric nanostructures, these biophotonic nanostructures achieve the large sizes necessary to scatter visible light. If engineers can learn to imitate the way insects create structural color, they may be able to improve the performance of light-manipulating devices, including fiber optics and solar cells.
Structural colors arise when multiple objects in a material scatter light, causing constructive or destructive interference of different wavelengths. These light scattering objects – which could be multilayers in a shell, air bubbles in a feather barb, or crystal-like arrays on a wing scale – work differently than molecular pigments that selectively absorb certain wavelengths while reemitting others. Few animal pigments can produce shorter wavelength blue and green colors Because of this, many insects rely on structural coloration to produce blues that will make them visible to potential mates and greens to conceal them from predators. However, structural colors in insects cover the entire spectrum, from violet to red.
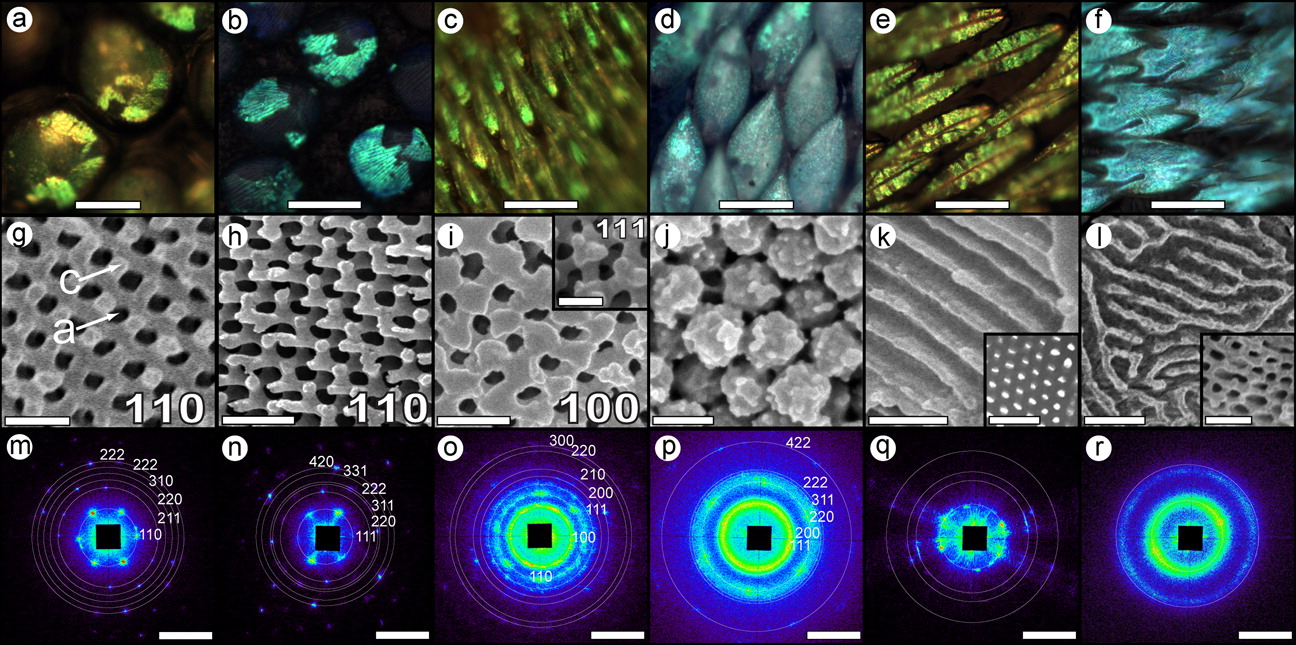
Although many studies have investigated structural colors in insects, the focus has typically been on one or a few species. Researchers from Yale University, Yale-NUS College (Singapore), CSIRO Ecosystem Sciences (Australia), and Argonne performed the first comprehensive survey of structural color in arthropods (the phylum that includes insects and spiders). Working at X-ray Science Division beamline 8-ID-I at the Argonne APS, which is an Office of Science user facility, as well as the SAXS/WAXS beamline of the Australian Synchrotron, and beamline I22 of the Diamond Light Source (UK), the team performed small-angle x-ray scattering (SAXS) measurements on 140 samples coming from nearly 100 species of longhorn beetles and weevils, several kinds of bees and spiders, and one butterfly. Butterflies structural colors were the subject of previous study by these authors using 8-ID-I (Saranathan et al., 2010, Proc. Nattl. Acad. Sci. USA, 107[26], 11676 [June 29, 2010], DOI: 10.1073/pnas.0909616107; see also: "What Makes Butterfly Wings Gleam?," APS Science 2010, pg. 56). Some of the specimens were collected more than a century ago, but their colors still remain vivid – a testament to the fact that structural colors don’t fade like pigment-based colors.
The nanostructures in the sample were sculpted within a single scale cell made of protein and chitin, a tough starchy material that insects and similar animals use for their outer skeleton. In the experiments, synchrotron light scatters off the interfaces between chitin and air. Since the sizes of these structures are roughly 100s of nanometers across, the short wavelength x-rays (roughly 0.1 nanometers) only scatter over very tiny angles (on the order of microradians). APS beamline 8-ID-I is one of the few facilities in the world that can record this small-angle scattering.
The SAXS data showed that the photonic nanostructures in insects and spiders appear in a wide variety of different morphologies, such as diamond lattices, single network gyroids, close-packed spheres, honey-combed columns, and sponge-like networks (see the figure). These morphologies are analogous to membrane structures that appear both in biology and industry. Certain molecules, like fatty lipid molecules in a cell or surfactants in detergents, can self-assemble into different nanoscale shapes. Engineers produce similar elements with long Lego-like molecules, called block copolymers, but the size of these artificial structures is limited by the length of the polymer chains ( no bigger than 50 nanometers). Insects assemble photonic nanostructures that are five to ten times larger than this, so maybe we can learn some design strategies by studying insect structural colors.
The authors note that cells produce several types of membrane shapes with the help of membrane-binding proteins. Perhaps insects have co-opted this molecular membrane-bending machinery to produce their photonic nanostructures. The researchers hypothesize that during insect development, membranes in the outer scale cells form a kind of nanostructure template, within which the biopolymer chitin fills in. When the scale cells eventually die, the chitin remains — shaped with color-producing nanostructures. If further work confirms this model, then it might be possible to design artificial molecules and membranes that can reproduce this self-assembly process.
Bio-inspired nanostructures could be used to better capture sunlight in a solar cell or to better channel light signals through an optical fiber.
— Michael Schirber
See: Vinodkumar Saranathan1,2,5*, Ainsley E. Seago3, Alec Sandy4, Suresh Narayanan4, Simon G.J. Mochrie5, Eric R. Dufresne5, Hui Cao5, Chinedum O. Osuji5, and Richard O. Prum5**, “Structural Diversity of Arthropod Biophotonic Nanostructures Spans Amphiphilic Phase-Space,” Nano Lett. 15(6), 3735 (2015). DOI: 10.1021/acs.nanolett.5b00201
Author affiliations: 1Nanyang Technological University, 2University of Oxford, 3CSIRO Ecosystem Sciences, 4 Argonne National Laboratory, 5 Yale University
Correspondence: *[email protected], **[email protected]
This work was supported with seed funding from the U.S. National Science Foundation (NSF) Materials Research Science and Engineering Center (DMR 1119826) and NSF grants to S.G.J.M. (DMR-0906697), H.C. (PHY-0957680); a Royal Society Newton Fellowship and Linacre College EPA Junior Research Fellowship to V.S., as well as Yale University W.R. Coe Funds to R.O.P. R.O.P. acknowledges the support of the Ikerbasque Science Fellowship and the Donostia International Physics Center. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.