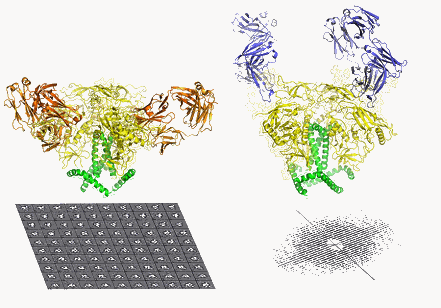
The first atomic-level structure of the tripartite HIV (human immunodeficiency virus) envelope protein—long considered one of the most difficult targets in structural biology and of great value for medical science—has been determined by scientists using data obtained at three synchrotron x-ray light sources including the U.S. Department of Energy (DOE) Office of Science’s Advanced Photon Source.
The new findings provide the most detailed picture yet of the acquired immune deficiency syndrome (AIDS)-causing virus’s complex envelope, including sites that future vaccines will try to mimic to elicit a protective immune response.
“Most of the prior structural studies of this envelope complex focused on individual subunits; but we’ve needed the structure of the full complex to properly define the sites of vulnerability that could be targeted, for example with a vaccine,” said Ian A. Wilson of The Scripps Research Institute (TSRI) and a senior author of the new research that included colleagues from TSRI; Weill Cornell Medical College; the Ragon Institute of MGH, MIT, and Harvard; and the Academic Medical Center (The Netherlands). The findings were published in two papers in Science Express, the early online edition of the journal Science, on October 31, 2013.
HIV currently infects about 34 million people globally, 10% of whom are children, according to World Health Organization estimates. Although antiviral drugs are now used to manage many HIV infections, especially in developed countries, scientists have long sought a vaccine that can prevent new infections and perhaps ultimately eradicate the virus from the human population.
However, none of the HIV vaccines tested so far has come close to providing adequate protection. This failure is due largely to the challenges posed by HIV’s envelope protein, known to virologists as Env.
HIV’s Env is not a single, simple protein but rather a “trimer” made of three identical, loosely connected structures with a stalk-like subunit, gp41, and a cap-like region, gp120. Each trimer resembles a mushroom and up to about 15 of these Env trimers sprout from the membrane of a typical virus particle, ready to latch onto susceptible human cells and facilitate viral entry.
Although Env in principle is exposed to the immune system, in practice it has evolved highly effective strategies for evading immune attack. It frequently mutates its outermost “variable loop” regions, for example, and also coats its surfaces with hard-to-grip sugar molecules (also called glycans).
Even so, HIV vaccine designers might have succeeded by now, had they been able to study the structure of the entire Env protein at atomic-scale—in particular, to fully characterize the sites where the most effective virus neutralizing antibodies bind. But Env’s structure is so complex and delicate that scientists have had great difficulty obtaining the protein in a form that is suitable for atomic-resolution imaging.
“It tends to fall apart, for example, even when it’s on the surface of the virus, so to study it we have to engineer it to be more stable,” said study co-author Andrew Ward of TSRI.
The key goal in this area has been to engineer a version of the Env trimer that has the stability and other properties needed for atomic-resolution imaging, yet retains virtually all the quaternary structure found on native Env.
After many years in pursuit of this goal, Moore, Rogier W. Sanders and their colleagues at Weill Cornell Medical College, working with Wilson, Ward, and others at TSRI, recently managed to produce a version of the Env trimer called BG505 SOSIP.664 gp140 that is suitable for atomic-level imaging work—and includes all of the trimer structure that normally sits outside the viral membrane.
The team then employed cutting-edge imaging methods to evaluate the new Env trimer. X-ray crystallography was carried out at the National Institute of General Medical Sciences and National Cancer Institute (GM/CA) structural biology beamlines 23-ID-D and 23-ID-B at Argonne National Laboratory’s Advanced Photon Source, at the DOE’s Stanford Synchrotron Radiation Lightsource, and at the Canadian Light Source. Electron microscopy was done at TSRI. The x-ray crystallography study was the first ever of an Env trimer, and both methods resolved the trimer structure to a finer level of detail than has been reported before.
“The new data are consistent with the findings on Env subunits over the last 15 years, but also have enabled us to explain many prior observations about HIV in structural terms for the first time,” said Jean-Philippe Julien, a senior research associate in the Wilson laboratory at TSRI, who was first author of the x-ray crystallography study.
The data illuminated the complex process by which the Env trimer assembles and later undergoes radical shape changes during infection and clarified how it compares to envelope proteins on other dangerous viruses, such as flu and Ebola.
Arguably the most important implications of the new findings are for HIV vaccine design. In both of the new studies, Env trimers were imaged while bound to broadly neutralizing antibodies against HIV. Such antibodies, isolated from naturally infected patients, are the very rare ones that somehow bind to Env in a way that blocks the infectivity of a high proportion of HIV strains. Ideally an HIV vaccine would elicit large numbers of such antibodies from patients, and to achieve that, vaccine designers would like to know the precise structural details of the sites where these antibodies bind to the virus—so that they can mimic those viral “epitopes” with the vaccine.
“It has been a privilege for us to work with the Scripps team on this project,” said John Moore on behalf of the Weill Cornell group. “Now we all need to harness this new knowledge to design and test next-generation trimers and see if we can induce the broadly active neutralizing antibodies an effective vaccine is going to need.”
“One surprise from this study was the revelation of the complexity and the relative inaccessibility of these neutralizing epitopes,” said Julien. “It helps to know this for future vaccine design, but it also makes it clear why previous structure-based HIV vaccines have had so little success.”
“We found that these neutralizing epitopes encompass features such as the variable loop regions and glycans that were excluded from previous studies of individual Env subunits,” said Dmitry Lyumkis, first author of the electron microscopy study, who is a graduate student at TSRI participating in the National Institutes of Health (NIH)-funded National Resource for Automated Molecular Microscopy. “We observed, too, that neutralizing antibody binding to gp120 can be influenced by the neighboring gp120 structure within the trimer—another complication that was not apparent when we were not studying the whole trimer.”
Having provided these valuable structural insights, the new Env trimer is now being put to work in vaccine development. “We aim to refine and increase the antibody response, then move forward to tests in humans,” said Ward.
See: Jean-Philippe Julien1, Albert Cupo2, Devin Sok1, Robyn L. Stanfield1, Dmitry Lyumkis1, Marc C. Deller1, Per-Johan Klasse2, Dennis R. Burton1,3, Rogier W. Sanders2,4, John P. Moore2*** Andrew B. Ward1**, and Ian A. Wilson1*, “ Crystal Structure of a Soluble Cleaved HIV-1 Envelope Trimer,” Published online ahead of print, Science Express, 31 October, 2013.
Author affiliations: 1The Scripps Research Institute; 2Weill Medical College of Cornell University; 3Ragon Institute of MGH, MIT, and Harvard; 4Academic Medical Center
Correspondence: *[email protected], **[email protected], ***[email protected]
This work was supported by NIH P01 AI82362 (J.P.M., I.A.W.), as well as the International AIDS Vaccine Initiative Neutralizing Antibody Consortium and Center (D.R.B., I.A.W., J.P.M., A.B.W.), CHAVI-ID UM1 AI100663 (D.R.B., I.A.W., AB.W.), NIH R01 AI084817 (I.A.W.), NIH R37 AI36082 (J.P.M.), NIH R01 AI33292 (D.R.B.), a Vidi grant from the Netherlands Organization for Scientific Research (R.W.S.), a Starting Investigator Grant from the European Research Council (R.W.S.), Canadian Institutes of Health Research fellowship (J.-P.J.), and the Ragon Institute. D.L. is supported by the U.S. NIH NIGMS Biomedical Technology Research Center program (GM103310). GM/CA has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104).
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under contract no. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The original Scripps Research Institute press release can be found here.
The Scripps Research Institute is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs about 3,000 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists—including three Nobel laureates—work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation.