Rheumatoid arthritis (RA) is a progressively incapacitating and devastating disease that involves destruction of many tissues within the body, but especially the joint tissues. About 1% of the world population is affected by RA, and the disease strikes women up to five times as often as men. RA is characterized by a highly variable disease course. Some of the afflicted will have mild and transient symptoms, but most will experience ongoing disease for the rest of their lives. Many different types of treatments can alleviate symptoms and/or modify the disease process; however, there is no known cure for rheumatoid arthritis and a need exists for therapies that will halt the underlying disease processes.
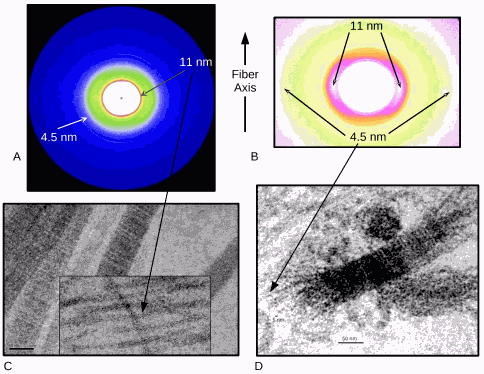
Joints contain cartilage, which consists of collagen fibers, and synovial tissue, which produces a fluid that helps lubricate the joints. Both the cartilage and synovial tissue are damaged in RA. The exact cause(s) of the destruction of joint tissues in RA have been unclear. Utilizing x-ray crystallography at an Advanced Photon Source x-ray beamline, researchers with the Illinois Institute of Technology were able to view the actions of an antibody targeted toward the proteoglycan biglycan — one of a group of polysaccharide-protein conjugates present in connective tissue and cartilage — that may help illustrate the underlying pathology of RA.
The role of x-ray crystallography was especially central to their work because the researchers were able to view the structure of antibody disrupted tissue without concern that sample preparation had introduced artifacts. The new findings pave the way for further studies; a greater understanding of the destructive process in RA may help in developing therapies that could ultimately prevent or delay joint disruption and help treat millions of patients with RA. The findings in part are based on x-ray diffraction data collected at the Biophysics Collaborative Access Team (Bio-CAT) 18-ID beamline at the Argonne Advanced Photon Source.
The current study speculates as to what happens to the joint cartilage collagen fibrils during RA. Proteins called proteoglycans bind to collagen and help to stabilize the collagen bundles and keep it healthy. In this study, utilizing x-ray crystallography and transmission electron microscopy (TEM), the researchers demonstrated that an antibody against a proteoglycan called biglycan resulted in tissue destruction that may be comparable to what actually happens in RA-affected tissues.
According to the researchers, elevated levels of anti-biglycan antibodies are detected in the body fluids of arthritis patients and are considered an early warning sign of the disease, but their specific role has not previously been clarified.
To test their theory about the antibody levels as early-warning signs, the researchers incubated thin pieces of cartilage with antibody for various time points and then visualized the extent of tissue disruption with TEM and x-ray diffraction experiments carried out at beamline 18-ID.
The x-ray diffraction data produced what has been described by these researchers as a plausible molecular mechanism for RA. According to the team, the destructive effect of the antibody was more potent than that of chemicals and other treatments often used to mimic RA tissue damage. The researchers suggest that the initiation of RA-associated tissue destruction during the actual disease process may involve a similar antibody-mediated breakdown of collagen fibrils that they observed in their study.
This proposed model highlights the crucial position of the biglycan antibody in the development of rheumatoid arthritis and the associated observations provide the first indication of what its role is beyond being a marker of this common and widespread disease. — Emma Hitt
See: Olga Antipova** and Joseph P.R.O. Orgel*, “Non-Enzymatic Decomposition of Collagen Fibers by a Biglycan Antibody and a Plausible Mechanism for Rheumatoid Arthritis.” PLoS ONE 7(3), e32241 (March2012). DOI:10.1371/journal.pone.0032241
Author affiliation: Illinois Institute of Technology
Correspondence: *[email protected], **[email protected]
This work was supported by the National Science Foundation (Grant #MCB-0644015 CAREER). This material is based on the work supported by, or in part by, the US Army Research Laboratory and the US Army Research Office under contract/grant number W911NF 09-1-0378. Bio-CAT is a National Institutes of Health-supported Research Center RR-08630. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.