In recent years, drug resistance has become an increasing problem in the treatment of tuberculosis (TB), posing a significant challenge for efforts to prevent and control this disease, which has been a leading cause of death worldwide for more than 5,000 years. Utilizing the U.S. Department of Energy Office of Science’s Advanced Photon Source, researchers unlocked the secrets of a protein that helps TB develop multidrug resistance. Understanding the results of this study will be important in guiding future research into new ways to control this disease.
Tuberculosis is a bacterial disease caused by Mycobacterium tuberculosis. It is easily transmitted through droplets in the air and predominantly affects the lungs. Although this is an ancient disease, it continues to pose significant problems to the public health. It is the leading cause of death from a curable infectious disease and is second only to human immunodeficiency virus/acquired immunodeficiency syndrome as the largest killer worldwide due to a single infectious agent.
TB is preventable and curable, but it is becoming more resistant to treatment. According to the World Health Organization, about 5% of cases are multidrug-resistant (MDR) – that is, they are resistant to isoniazid and rifampicin, two drugs that are first-line treatments for this condition.
The problem has further spiraled out of control as additional antibiotics have been used to attempt to control the situation, resulting in extensively drug-resistant TB, which is harder to control, and most recently, totally drug-resistant TB, which appears to be untreatable.
A better understanding of the molecular mechanisms of drug resistance in M. tuberculosis is therefore important for the continued development of new strategies to control this disease.
Recent studies have suggested that MDR strains of M. tuberculosis are associated with bacterial transporter pumps that, when switched on, can work to actively flush out a range of compounds from within the bacterial cells. This includes the very medications that are being used to target the organism. One of these pumps is the Mmr multidrug efflux pump, which is involved in the development of multidrug resistance. Because very little has been known about what regulates the Mmr pump, this study aimed to investigate this mysterious biological device.
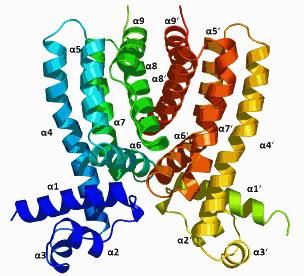
Utilizing the Northeastern Collaborative Access Team x-ray beamline 24-ID-E at the Argonne APS, researchers from Iowa State University and Cornell University determined the crystal conformation of the regulator protein Rv3066 (Fig. 1).
An important finding of their study provides insight into the structure and function of this regulator protein.
This protein, which regulates the Mmr bacterial pump, is an asymmetric, two-part regulator molecule with a flexible, spiral structure that allows it to recognize and respond to drugs.
The researchers found that in the presence of ethidium bromide, a toxic compound and substrate transported by the pump, the regulator changes its conformation. It undergoes a structural rotation, thereby switching on the pump that effectively gets rid of antimicrobial drugs from the bacterial organism.
Although further details behind the mechanism of multidrug resistance in TB remain to be determined, studying the structure of this regulator and how it acts to control the bacterial pump will lead to a better understanding of how the bacterium contributes to multidrug resistance, and how it can change according to its environment.
These results will be important in guiding future research in the continued development of new ways to control TB, and could make a big difference in the fight against drug-resistant forms of the condition. — Nicola Parry
See: Jani Reddy Bolla1, Sylvia V. Do1, Feng Long1, Lei Dai1, Chih-Chia Su1, Hsiang-Ting Lei1, Xiao Chen1, Jillian E. Gerkey1, Daniel C. Murphy1, Kanagalaghatta R. Rajashankar2, Qijing Zhang1, and Edward W. Yu1*, “Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis, Nucleic Acids Research, First published online July 19, 2012. DOI:10.1093/nar/gks677
Author affiliations: 1Iowa State University, 2Cornell University
Correspondence: *[email protected]
This work is supported by National Institutes of Health (NIH) DK063008 to Q.Z. and GM086431 to E.W.Y., a Community College Institute of Science Internship (CCI), and a Science Undergraduate Laboratory Internship (SULI) from the U.S. Department of Energy to J.E.G. and D.C.M.
The Northeastern Collaborative Access Team is funded by Columbia University Cornell University, Harvard University, the Memorial Sloan-Kettering Cancer Center, the Massachusetts Institute of Technology, Rockefeller University, Yale University, and the National Institute of General Medical Science.
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy’s Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.