Smart viruses find ways around host defenses. In the case of the influenza viruses A and B, rapid genetic changes and resistance to available therapies make it hard to combat flu epidemics in humans. Mortality rates for influenza B viruses are higher than those reported for seasonal influenza A H1N1. Human monoclonal antibodies were previously shown to neutralize a wide variety of influenza A viruses, so extending that work to include influenza B viruses is critical because these flu strains cause a large proportion of the annual flu infections and are a major cause of seasonal epidemics every two to four years.
A research group from The Scripps Research Institute, Crucell Vaccine Institute, Gustav Wieds Vej (Denmark), and The University of Hong Kong, building upon their earlier work with influenza A viruses, has now discovered a similar phenomenon for neutralizing influenza B viruses. Their breakthrough results, aided by the U.S. Department of Energy (DOE) Office of Science’s Advanced Photon Source (APS), Stanford Synchrotron Radiation Lightsource (SSRL), and Advanced Light Source (ALS) pave the way for development of a universal vaccine for all influenza A and B viruses.
“Several structures were solved using the [GM/CA CAT 23-ID-B beamline] facilities [at the APS] in order to study the interaction of CR9114 with group 2 influenza A viruses,” said Cyrille Dreyfus, lead author on the paper and team member from The Scripps Research Institute. “These structures allowed us to confirm the broad neutralizing activity of CR9114”.
The earlier work on the influenza A viruses showed that monoclonal antibodies can neutralize the viruses by binding to a highly conserved region, called an epitope, in the stem region of a viral surface glycoprotein. To look for similar reactions in the influenza B virus, the research team focused on two co-circulating, antigenically distinct strains.
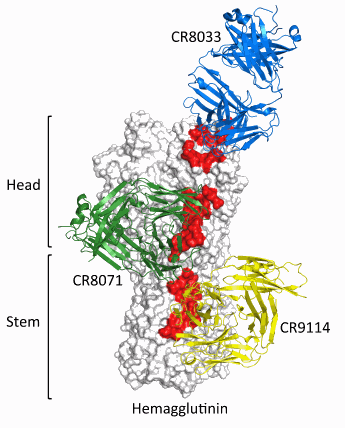
The researchers found three human monoclonal antibodies that protect against potentially lethal infection from these two influenza B strains. Two of the antibodies, CR8033 and CR8071, bind to conserved epitopes in the head region of the influenza B hemagglutinin (HA). The third antibody, CR9114, attaches to a conserved epitope in the HA stem, and, most important, provides protection against influenza A and B viruses.
The study showed that the three antibodies did not compete against each other for HA binding sites and thereby concluded that they recognized different epitopes. The results pointed to the mechanism by which the antibodies CR8033 and CR8071 thwarted viral infection: although the virus gained entry to the cell and performed genome replication, propagation of the virus was prevented.
Antibodies CR8033 and CR8071 appear to prevent release of viral progeny from infected cells and, hence, disrupt the infection process. CR9114 uses a different mechanism and blocks infection by preventing the low pH conformational changes that trigger the fusion of the viral and endosomal membranes during the entry process (Fig. 1).
A key aspect of these results is the finding that the CR9114 epitope appears to be highly conserved in all influenza A subtypes and in influenza B. This finding is so important primarily because it provides an inroad to the previously intractable problem of finding a universal influenza vaccine. A highly conserved epitope is likely to remain the same even if the virus undergoes repeated mutations and resistance cycles. And when searching for a vaccine, a neutralizing epitope region that remains constant and therefore vulnerable to existing treatments is a beautiful thing. Antibodies such as CR9114 may be possible to elicit by vaccination and may already be present in the immune systems of some individuals.
“Future directions,” said Dreyfus, “are to apply this knowledge toward the development of improved therapies and vaccines such as development of monoclonal antibody therapies,” and “the design of small molecules that target the weak point in the virus defense.”
Since previous work had shown that the influenza A viruses exhibited broadly neutralizing epitopes in the HA stem, those now found in the influenza B HA head point to both the head and stem regions of these viruses as targets for neutralizing antibodies.
Universal therapies against the many strains of influenza that may be circulating at any given time require somewhat of a “one vaccine fits all” approach. The work presented in this research study — especially that concerning the CR9114 antibody — brings that dream into the realm of the possible. — Mona Mort
See: Cyrille Dreyfus1, Nick S. Laursen1,2, Ted Kwaks3, David Zuijdgeest3, Reza Khayat1, Damian C. Ekiert1†, Jeong Hyun Lee1, Zoltan Metlagel1‡, Miriam V. Bujny3, Mandy Jongeneelen3, Remko van der Vlugt3, Mohammed Lamrani3, Hans J. W. M. Korse3, Eric Geelen3, Özcan Sahin3, Martijn Sieuwerts3, Just P. J. Brakenhoff3, Ronald Vogels3, Olive T. W. Li4, Leo L. M. Poon4, Malik Peiris4, Wouter Koudstaal3, Andrew B. Ward1, Ian A. Wilson1*, Jaap Goudsmit3**, and Robert H. E. Friesen3, “Highly Conserved Protective Epitopes on Influenza B Viruses,” Science 337, 1343 (14 September 2012). DOI:10.1126/science.1222908
Author affiliations: 1The Scripps Research Institute, 2Gustav Wieds Vej, 3Crucell Vaccine Institute, 4The University of Hong Kong • Present addresses: †University of California, San Francisco. ‡Lawrence Berkeley National Laboratory
Correspondence: * [email protected]; ** [email protected]
This project has been funded in part by the Area of Excellence Scheme of the University Grants Committee, Hong Kong (grant AoE/M-12/06); a predoctoral fellowship from the Achievement Rewards for College Scientists Foundation (D.C.E.); grant GM080209 from the National Institutes of Health (NIH) Molecular Evolution Training Program (D.C.E.); a Saper Aude Postdoc grant from the Danish Council for Independent Research, Natural Sciences (N.S.L.), and the Skaggs Institute (I.A.W.). Portions of this research were carried out at the SSRL, a national user facility operated by Stanford University on behalf of the DOE Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by NIH, National Center for Research Resources, Biomedical Technology Program (P41RR001209), and the National Institute of General Medical Sciences (NIGMS). The ALS is supported by the director, Office of Science, Office of Basic Energy Sciences, of the DOE under Contract No. DE-AC02-05CH11231.The GM/CA 23-ID-B beamline at the APS has been funded in whole or in part with federal funds from National Cancer Institute (Y1-CO-1020) and NIGMS (Y1-GM-1104).
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. DOE Office of Science under Contract No. DE-AC02-06CH11357
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by U. Chicago Argonne, LLC for the U.S. Department of Energy's Office of Science.