Computer-designed proteins are under construction to fight the flu. Researchers who carried out studies at the U.S. Department of Energy Office of Science’s Advanced Photon Source at Argonne National Laboratory are demonstrating that proteins that are found in nature, but do not normally bind the influenza virus, can be engineered to act as broad-spectrum antiviral agents against a variety of flu virus strains, including the H1N1 pandemic influenza.
"One of these engineered proteins has a flu-fighting potency that rivals that of several human monoclonal antibodies," said David Baker, professor of biochemistry at the University of Washington, in a report in Nature Biotechnology.
The research team in this study, from the University of Washington, The Scripps Research Institute, and the Naval Health Research Center is making major inroads in optimizing the function of computer-designed influenza inhibitors. These proteins are constructed via computer modeling to fit exquisitely into a specific nano-sized target on flu viruses. By binding the target region like a key into a lock, they keep the virus from changing shape, a tactic that the virus uses to infect living cells. The research efforts, akin to docking a space station but on a molecular level, are made possible by computers that can describe the landscapes of forces involved on the submicroscopic scale.
Because influenza is a serious worldwide public health concern due to its genetic shifts and drifts that periodically become more virulent, the flu is one of the key interests of many researchers, including those at the Institutes for Protein Design at the University of Washington and its collaborators in the United States and abroad. Researchers are trying to meet the urgent need for better therapeutics to protect against this very adaptable and extremely infective virus. Vaccines for new strains of influenza take months to develop, test and manufacture, and are not helpful for those already sick. The long response time for vaccine creation and distribution is unnerving when a more deadly strain suddenly emerges and spreads quickly. The speed of transmission is accelerated by the lack of widespread immunity in the general population to the latest form of the virus.
Flu trackers refer to strains by their H and N subtypes. H stands for hemagglutinins, which are the molecules on the flu virus that enable it to invade the cells of respiratory passages. The virus's hemagglutinin molecules attach to the surface of cells lining the respiratory tract. When the cell tries to engulf the virus, it makes the mistake of drawing it into a more acidic location. The drop in pH changes the shape of the viral hemagglutinin, thereby allowing the virus to fuse to the cell and open an entry for the virus' RNA to come in and start making fresh viruses. It is hypothesized that the Baker Lab protein inhibits this shape change by binding the hemagglutinin in a very specific orientation and thus keeps the virus from invading cells.
The research team wanted to create antivirals that could react against a wide variety of H subtypes, as this versatility could lead to a comprehensive therapy for influenza. Specifically, viruses that have hemagglutinins of the H2 subtype are responsible for the deadly pandemic of 1957 and continued to circulate until 1968. People born after that date haven't been exposed to H2 viruses. The recent avian flu has a new version of H1 hemagglutinin. Data suggests that Baker's proteins bind to all types of the Group I Hemagglutinin, a group that includes not just H1 but the pandemic H2 and avian H5 strains.
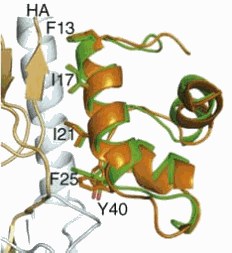
Recognizing the importance of new flu therapies to national and international security, the Defense Advanced Research Projects Agency and the Defense Threat Reduction Agency funded this work, along with the National Institutes of Health's National Institute for Allergy and Infectious Diseases. The researchers used the National Institute of General Medical Sciences and National Cancer Institute Structural Biology Facility 23-ID-B x-ray beamline at the APS to determine he x-ray structure of F-HB80.4 in complex with the SC1918/H1 hemagglutinin ectodomain at 2.7-Å resolution.
The methods developed for the influenza inhibitor protein design, Baker said, could be "a powerful route to inhibitors or binders for any surface patch on any desired target of interest." For example, if a new disease pathogen arises, scientists could figure out how it interacts with human cells or other hosts on a molecular level. Scientists could then use protein interface design to generate a diversity of small proteins that they predict would block the pathogen's interaction surface.
Genes for large numbers of the most promising, computer-designed proteins could be tested using yeast cells. After further molecular chemistry studies to find the best binding among those proteins, those could be re-programmed in the lab to undergo mutations, and all the mutated forms could be stored in a "library" for an in-depth analysis of their amino acids, molecular architecture and energy bonds. Advanced technologies would allow the scientists to quickly thumb through the library to pick out those tiny proteins that clung to the pathogen surface target with pinpoint accuracy. The finalists would be selected from this pool for excelling at stopping the pathogen from attaching to, entering and infecting human or animal cells.
The use of deep sequencing, the same technology now used to sequence human genomes cheaply, was especially crucial in creating detailed maps relating sequencing to function. These maps were used to reprogram the design to achieve a more precise interaction between the inhibitor protein and the virus molecule. It also enabled the scientists, they said, "to leapfrog over bottlenecks" to improve the activity of the binder. They were able to see how small contributions from many tiny changes in the protein, too difficult to spot individually, could together create a binder with better attachment strength.
"We anticipate that our approach combining computational design followed by comprehensive energy landscape mapping," Baker said, "will be widely useful in generating high-affinity and high-specificity binders to a broad range of targets for use in therapeutics and diagnostics."
See: Timothy A. Whitehead1,‡, Aaron Chevalier1, Yifan Song1, Cyrille Dreyfus2, Sarel J. Fleishman1, ‡ ‡, Cecilia De Mattos3, Chris A. Myers3, Hetunandan Kamisetty1, Patrick Blair3, Ian A. Wilson2, and David Baker1,*, “Optimization of affinity, specificity and function of designed influenza inhibitors using deep sequencing,” Nat. Biotech. 30(6), 543 (6 June 2012). DOI:10.1038/nbt.2214
Author affiliations: 1University of Washington, 2The Scripps Research Institute, 3Naval Health Research Center. Present addresses: ‡Michigan State University, ‡ ‡Weizmann Institute of Science
Correspondence: *[email protected]
This work was funded by Defense Advanced Research Projects Agency (DARPA) and the Defense Threat Reduction Agency (DTRA), and U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Institute of General Medical Sciences. Use of the GM/CA facility has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. DE-AC02-06CH11357.
The original University of Washington press release can be found here.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.