Sometimes nature cannot be improved upon. One example is in the synthesis of nanomaterials, which in the laboratory or factory generally requires toxic chemicals and extreme conditions of temperature and pressure. But over millions of years, nature has developed ways of putting together inorganic nanocrystals at mild temperatures and pressures. Usually this process, known as biomineralization, involves calcium carbonate or phosphate for purposes such as building bone or shells, but another interesting variation is seen in the crystallization of gold from solution by certain types of bacteria. A group of researchers has devised a unique experiment to mimic this natural process of biomineralization in order to create oriented gold nanocrystals and examine their formation at the U.S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory.
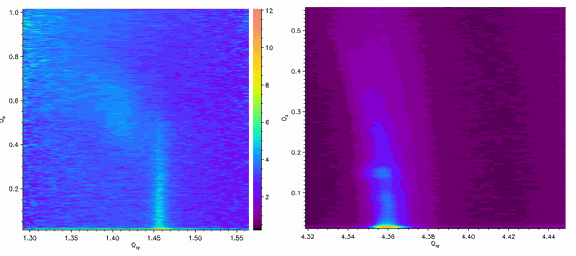
Working at the ChemMatCARS 15-ID beamline at the APS, the researchers from Northwestern University and the University of Chicago floated Langmuir monolayers of octadecanethiol (C18S) on solutions of chloroauric acid (HAuCl4) at room temperature and pressure, then employed a monochromatic 10 keV x-ray beam both as a reduction agent to induce gold crystallization and as a probe to examine the interface through grazing-incidence x-ray diffraction (GID, Fig. 1). (The experiments were repeated at sector X14A of National Synchrotron Light Source to confirm that the results were not artifacts of the setup or the beam.)
“Self-assembly of organic molecules is well-known and well-studied on gold surfaces, and we wanted to use this knowledge from that field to grow gold nanoparticles by using an organic template,” says Ahmet Uysal, first author of the Physical Review Letters article on the group’s result.
By covering the undersurface of the floating Langmuir monolayer with gold, the experimenters essentially reversed the SAM (self-assembled monolayer) creation process and used it as an analogue for biomineralization. Co-author Pulak Dutta noted: “Alkylthiol SAMs have a structure perfectly matching the (111) face of gold. Inspired by this, we made Langmuir monolayers on aurochloric acid solutions, and then we grew gold crystals under them by using x-rays to reduce the gold.”
In doing so, added Uysal, “we can see the molecular interactions at the interface, how the organic molecule structures change during the process, and also the surface structures of the gold nanoparticles at the same time. Instead of trial and error methods to grow gold nanoparticles, we can see the process going on at the nanoscale.” The work offers important insights into the actual molecular interactions.
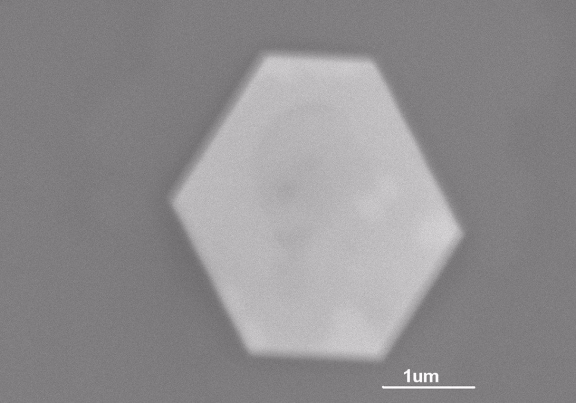
The GID peaks reveal that gold crystals formed at the thiol surface, with a (111) orientation commensurate with the organic template. Samples of the gold crystals were imaged with transmission electron microscopy (TEM), showing plate-like hexagonal nanocrystals about 50 nm wide (Fig. 2). The thiol monolayer behaves as a soft template, changing itself to accommodate formation of the nanocrystals.
It is this adaptability of the monolayer that promotes the growth of the oriented gold nanoparticles. “The fact that we can ‘trick’ gold into growing in a crystallographically oriented way is the major news in this paper,” Dutta points out. “Just as with SAMs, the structure of the organic monolayer matches the structure of the gold surface, and this lattice match makes gold crystals want to grow with all the (111) planes pointed the same way.”
By showing a method by which organic molecules can be used to control the shape, size, and crystallographic orientation of inorganic nanocrystals in a mild environment, the researchers have opened a path for the development of improved manufacturing processes for nanomaterials. Although current techniques using high temperatures and hard vacuum provide large yields, they are also more expensive and less environmentally friendly. Uysal explains, “Understanding the basics of the interaction may help to increase the yield of these more ‘green’ methods.” Dutta adds that “this is a process that happens in normal conditions. It is true that x-rays are used to reduce the gold, but such reduction can also be done chemically, which is how bacteria do it.”
The next step, says Uysal, is “to quantify the role of the chemistry and the structure of the monolayer in gold nanoparticle orientation and shape. There are other functional groups in living organisms such as amine and carboxyl groups. We want to see what works and what doesn’t. The ultimate goal is, of course, to be able to design templates for desired nanoparticle shapes and orientations.” Dutta adds, “By being smart about putting the right molecules on the template, we should be able to make better materials for photonics or other purposes.” — Mark Wolverton
See: Ahmet Uysal1, Benjamin Stripe1, Binhua Lin2, Mati Meron2, and Pulak Dutta1, “Reverse Self-Assembly: (111)-Oriented Gold Crystallization at Alkylthiol Monolayer Templates,” Phys. Rev. Lett. 107, 115503 (2011).
DOI: 10.1103/PhysRevLett.107.115503
Author affiliations: 1Northwestern University, 2The University of Chicago
Correspondence: * [email protected]
This work was supported by the U.S. National Science Foundation under Grant No. DMR-1006432. ChemMatCARS Sector 15 is principally supported by the National Science Foundation/Department of Energy under grant number NSF/CHE-0822838. Beamline X14A and the National Synchrotron Light Source are supported by the U.S. DOE. The electron micrographs were recorded at Electron Probe Instrumentation Center, Northwestern University.
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the DOE’s Office of Science under Contract No. DE-AC02-06CH11357.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science x-ray user facilities, visit http://science.energy.gov/user-facilities/basic-energy-sciences/.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.