Is the presence of arsenic in the environment, whether naturally occurring or as a result of human activity, a cause for concern? After all, humans eat dirt (children ingest an average of 80 milligrams of dirt a day, four times as much as adults). What happens when dirt contains arsenic? Detailed studies of the various forms of arsenic present in mine-impacted soils, examined using two U.S. Department of Energy Office of Science (DOE-SC) synchrotron facilities, have enabled researchers to establish a direct correlation between the compounds and the bioaccessibility of the arsenic they contain, pointing to new ways of assessing the risk factor associated with arsenic in the environment.
The bioaccessibility of arsenic—its ability to solubilize in the gastrointestinal tract, thus becoming available for absorption—depends on how arsenic is hosted in soil. According to Kenneth Reimer, lead researcher and director of the Environmental Sciences Group (ESG) at the Royal Military College of Canada, “People tend to get frightened when they hear about chemicals in the environment, but those chemicals have to get in the bloodstream first [in order to do any damage]. The default assumption is that all arsenic attached to ingested particles will enter your system, but you're going to eliminate some of it through waste.” The new methodology developed by the ESG and their colleagues provides a link between the bioaccessibility of arsenic and its mineral forms by examining the detailed mineralogy and speciation of the arsenic in contaminated soils using synchrotron techniques.
Utilizing beamline 20-BM at the DOE-SC’s Advanced Photon Source (APS) at Argonne, and beamline X26A of DOE-SC’s National Synchrotron Light Source at Brookhaven National Laboratory, Reimer’s group utilized x-ray absorption near edge structure (XANES), the micro-x-ray diffraction, and the micro-x-ray fluorescence techniques to examine soil and tailings samples taken from abandoned gold mines in Nova Scotia. The XANES measurements enable the determination of arsenic oxidation state and local environment, that is, whether the arsenic is coordinated by oxygen or sulphur. With microfluorescence, the elemental composition of a single grain in the soil can be examined, and with microdiffraction, crystalline structure can be identified and compared to known minerals.
In the Nova Scotia samples, ore processing and subsequent weathering have transformed the arsenic originally present, resulting in complex mixtures of various forms of arsenic, each with their own characteristics. The detailed mineralogy of each sample therefore varies, which affects its bioaccessibility. “It's the work with the synchrotrons that made this kind of experiment a success,” said Reimer. “We couldn't have done it without the two synchrotrons.” Either synchrotron could have been used for all the work in this study, but the ready access afforded by the user programs at each facility allowed the group to move ahead with their research.
The bioaccessibility tests run at the ESG facility in Canada only determine how much arsenic will dissolve, not the form in which the arsenic was originally present. The x-ray measurements at the DOE-SC synchrotron facilities only determine the chemical form of the arsenic present in the soil, but not what effect digestion may have on it. The breakthrough achieved by Reimer’s Ph.D. student Louise Meunier is in recognizing the strong, empirical correlation between test and measurement.
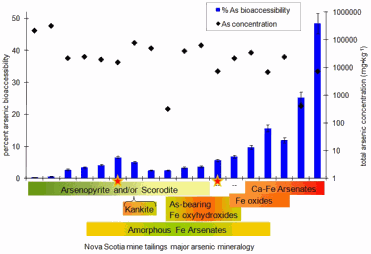
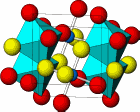
Taken together, the research at the ESG facility and at the two light sources reveal that certain arsenic minerals, such as arsenopyrite, have lower bioaccessibility; more than 90% of the arsenic in the sample would pass harmlessly through the digestive system without being absorbed by the body. This is due to arsenopyrite's low solubility, crystalline structure, and inability to dissolve in gastrointestinal fluids. Arsenic bearing iron(oxy)hydroxides (ferrihydrite, the structure of which was recently determined using XSD beamline 11-ID at the APS) and calcium-iron arsenates however, being much more soluble compounds, had 10 times the bioaccessibility of arsenopyrite.
Despite knowing the bioaccessibility of identified minerals in a sample, researchers still needed a bioaccessibility test of the entire sample to reveal higher-than-expected potential risks.
“This has been quite an insight for us,” Reimer said. “At the end of the day, you have to run the bioaccessibility test.” Nonetheless, mineralogical analysis has its own uses. “Mineralogy is helpful in the case of unmixed minerals' bioaccessibility,” Reimer said. In addition, the processes developed for this study can be applied to test the bioaccessibility of samples with a lower concentration of arsenic. Risk assessments can then include these results.
See: L. Meunier1, S.R. Walker2, J. Wragg3, M.B. Parsons4, I. Koch1, H.E. Jamieson2, and K.J. Reimer1*, “Effects of Soil Composition and Mineralogy on the Bioaccessibility of Arsenic from Tailings and Soil in Gold Mine Districts of Nova Scotia,” Environ. Sci. Technol. 44(7), 2667 (2010). DOI:10.1021/es9035682
Author affiliations: 1Royal Military College, 2Queen’s University, 3British Geological Survey, 4Natural Resources Canada, Geological Survey of Canada (Atlantic)
Correspondence: *[email protected]
The authors gratefully acknowledge the support of the National Science and Engineering Research Council via the Metals in The Human Environment Strategic Network (a full list of sponsors at www.mithe-sn.org) and a Discovery Grant (to K.J.R.) and the APS PNC/XOR, in particular R. Gordon. The Sector 20 facilities at the APS, and research at these facilities, are supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, a major facilities access grant from NSERC, the University of Washington, Simon Fraser University, and the APS. Beamline X26A, National Synchrotron Light Source, Brookhaven National Laboratory, is supported by the U.S. DOE-Geosciences (DE-FG02-92ER14244 to The University of Chicago - CARS) and the Office of Biological and Environmental Research, Environmental Remediation Sciences Division (DE-FC09-96-SR18546 to the University of Georgia). Use of NSLS was supported by DOE under Contract DEAC02-98CH10886. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357.
The original Brookhaven National Laboratory news release can be found here: http://www.nsls.bnl.gov/newsroom/science/2010/09-422.asp
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science, Office of Basic Energy Sciences (DOE-BES). The APS is the source of the Western Hemisphere’s brightest high-energy x-ray beams for research in virtually every scientific discipline. More than 3,500 scientists representing universities, industry, and academic institutions from every U.S. state and several foreign nations visit the APS each year to carry out applied and basic research in support of the BES mission to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels in order to provide the foundations for new energy technologies and to support DOE missions in energy, environment, and national security. To learn more about the Office of Basic Energy Sciences and its x-ray user facilities, visit .
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.