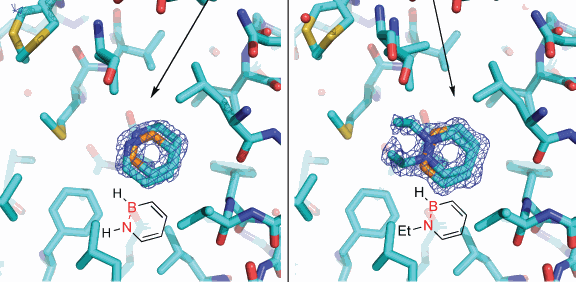
Chemists and biologists have successfully demonstrated that specially synthesized boron compounds are readily accepted in biologically active enzymes, a move that, they say, is a proof of concept that could lead to new drug design strategies.
In June 2008, University of Oregon chemist Shih-Yuan Liu reported in the Journal of the American Chemical Society his lab's synthesis of boron-nitrogen compounds with electronic and structural similarities to fundamentally important benzene molecules. That synthesis suggested a new tool for possible use in biomedical research as well as in materials science.
Liu had created benzene surrogates known as 1,2-dihydro-1,2-azaborines that possess electron-delocalized structures consistent with aromaticity — a core concept in chemistry where rings of atoms exhibit unexpected stability.
Now, Liu and colleagues from the University of Oregon have shown that their synthesized compounds are, indeed, accepted in non-polar hydrophobic pockets of a well-studied enzyme, a member of the lysozyme family discovered by Alexander Fleming in 1921 and used widely in biomedical research. The proof of concept was completed with the help of high-resolution x-ray crystallography carried out at the General Medicine and Cancer Institutes Collaborative Access Team x-ray beamline 23-ID at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source at Argonne National Laboratory, and x-ray beamline 5.0.2 at the DOE’s Advanced Light Source at Lawrence Berkeley National Laboratory. The results were published in Angewandte Chemie International Edition.
"I feel this is a fairly big step forward," Liu said. "Our compounds bind efficiently to the T4 lysozyme and behave as hydrophobic arene molecules similar to natural systems. Our compound actually has polar features, so it was questionable that it would bind to the enzyme's hydrophobic pocket, but it did and very similarly to the way carbon molecules would bind."
In essence, the researchers have potentially put boron, a commonly occurring essential nutrient in plants — but seemingly "bypassed by nature in evolution" of other living things, Liu said — into play as an alternative to carbon in manufacturing target-specific pharmaceuticals. The use of boron in the biomedical field is not new; its acceptance has been hampered by instability, but interest has risen in the last decade, Liu said.
An analysis of boron's medical potential appeared in the February issue of EMBO Reports. Boron is being studied by a number of drug manufacturers and is currently used as an antibacterial drug component and as part of a therapy for multiple myeloma. The advance by Liu's lab strengthens the case that boron-based molecules can be used as new pharmacophores, or as markers of drugs in living tissue, and to improve long-stymied attempts to develop boron-neutron capture therapies to produce inhibiting agents for cancer treatment.
"This research provides the first experimental evidence that enzymes in our bodies cannot distinguish between our artificial compound versus the all-carbon systems," Liu said. "We can trick the enzymes into believing they are accepting the real thing."
See: Lijun Liu, Adam J. V. Marwitz, Brian W. Matthews, and Shih-Yuan Liu*, “Boron Mimetics: 1,2-Dihydro-1,2-azaborines Bind inside a Nonpolar Cavity of T4 Lysozyme,” Angew. Chem. Int. Ed. 121(37), 6949, September 1, 2009. DOI: 10.1002/ange.200903390.
Author affiliation: University of Oregon
Correspondence: *[email protected]
Support has been provided by the University of Oregon and National Institutes of Health grant GM21967.
The original news release can be found at: https://uonews.uoregon.edu/archive/news-release/2009/9/boron-based-compounds-trick-biomedical-protein
Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.